ActiveNews se confruntă cu cenzura pe rețele sociale și pe internet. Intrați direct pe site pentru a ne citi și abonați-vă la buletinul nostru gratuit. Dacă doriți să ne sprijiniți, orice donație este binevenită. Doamne, ajută!
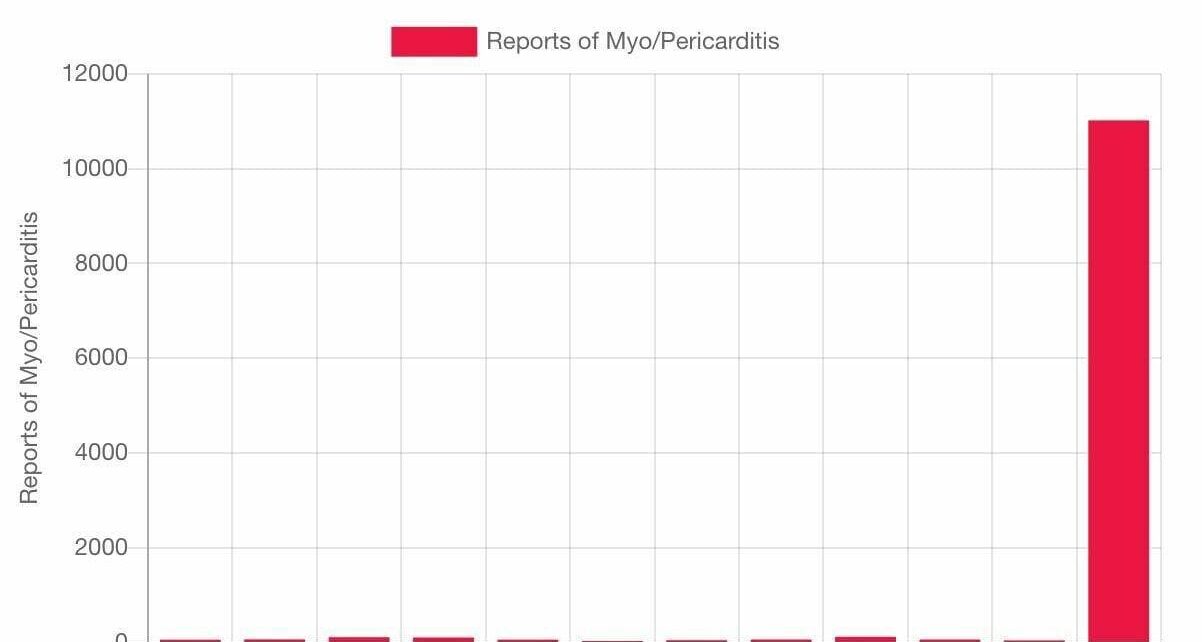
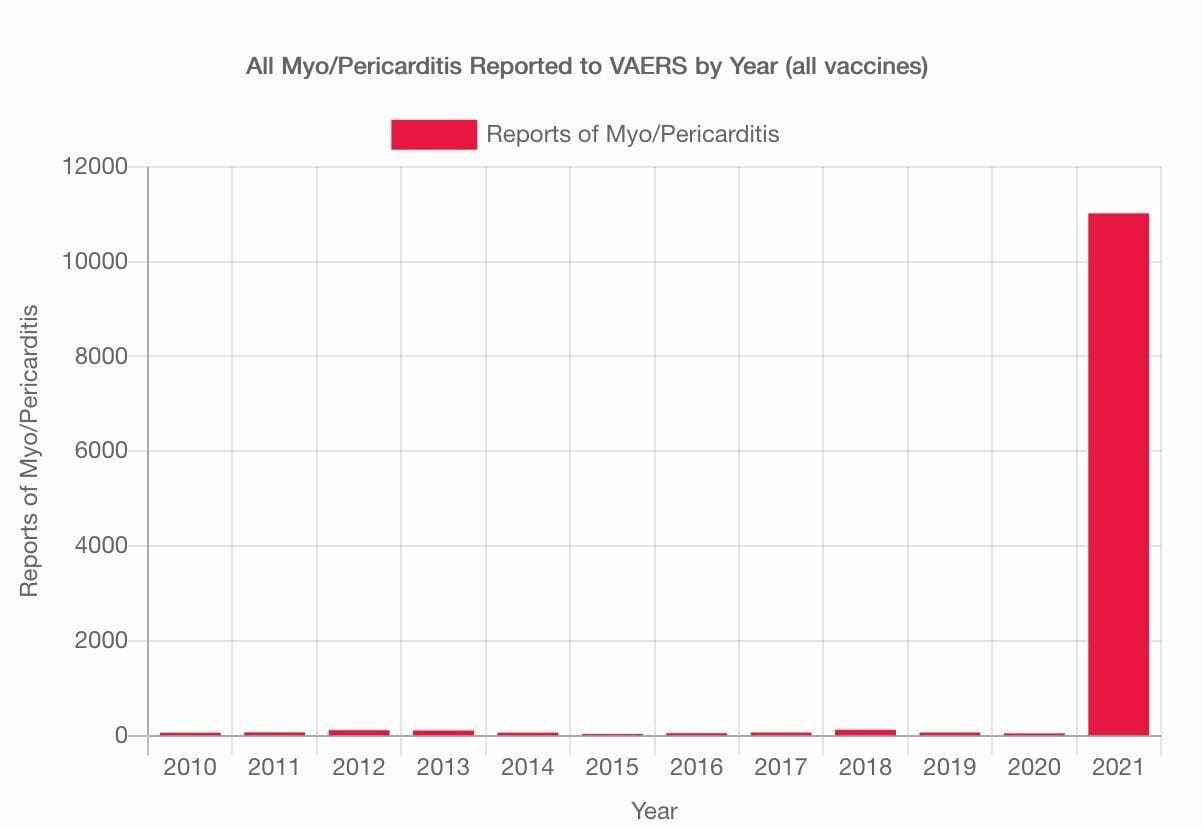
O studentă la medicină din România ne-a trimis cercetarea sa despre efectele adverse ale vaccinurilor Covid, miocradite și pericardite, tromboze și multe altele, cu gândul că ar putea fi de folos savanților de renume dâmbovițean care nu au produs nici unul un astfel de studiu, dar mai ales părinților și celor de vârsta ei, a tinerilor. Iată, tinerii de azi sunt mai deștepți decât propagandiștii Ro Vaccinare. Nici n-ar avea cum altfel, dacă șeful Ro Vacciniștilor crede că miocarditele afectează două organe – și cordul și inima. Iată cercetarea ei impresionantă, pentru care îi mulțumim.
La doar 1 an de la inceperea campaniei de vaccinare anti-covid, in
SUA si UE s-au inregistrat oficial mai multe milioane de efecte
secundare grave si mai multe zeci de mii de decese.
Au fost publicate mii de studii medicale, care arata ca
vaccinurile nu sunt nici SAFE si nici Effective. Nu sunt sigure,
pentru ca pot cauza numeroase probleme de sanatate-boli cardio
vasculare grave- miocardite, pericardite, cheaguri de sange,
tromboze, pot cauza boli neurologice grave, inclusiv pareze,
sindromul Guiallan Barre si Scleroza Mutipla, pot cauza cancere,
infertilitate, dereglarea sistemului imunitar. Vaccinurile nu sunt
nici eficiente, deoarece nu previn imbolnavirea si nici transmiterea
virusului.
In aceste conditii, in care exista studii stiintifice oficiale
despre periculozitatea acestor vaccinuri, fortarea populatiei sa se
vaccineze, ingradirea drepturilor si libertatilor fundamentale si
persecutarea celor care refuza vaccinarea, este echivalenta cu
GENOCIDUL PREMEDITAT, pe langa abuzul in serviciu si tentativa de
lovitura de stat.
Publicam aici o lista cu peste 1000 de studii stiintifice despre
efectele adverse grave cauzate de vaccinurile anti-covid. Va invitam sa selectati Dvs linkurile pentru accesare directa.
Cerebral venous thrombosis after COVID-19 vaccination in the
UK: a multicentre cohort study:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01608-1/
Vaccine-induced immune thrombotic thrombocytopenia with
disseminated intravascular coagulation and death after ChAdOx1
nCoV-19 vaccination:
https://www.sciencedirect.com/science/article/pii/S1052305721003414
Fatal cerebral hemorrhage after COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33928772/
Myocarditis after mRNA vaccination against SARS-CoV-2, a case
series:
https://www.sciencedirect.com/science/article/pii/S2666602221000409
Three cases of acute venous thromboembolism in women after
vaccination against COVID-19:
https://www.sciencedirect.com/science/article/pii/S2213333X21003929
Acute thrombosis of the coronary tree after vaccination
against COVID-19:
https://www.sciencedirect.com/science/article/abs/pii/S1936879821003988
US case reports of cerebral venous sinus thrombosis with
thrombocytopenia after vaccination with Ad26.COV2.S (against
covid-19), March 2 to April 21, 2020:
https://pubmed.ncbi.nlm.nih.gov/33929487/
Portal vein thrombosis associated with ChAdOx1 nCov-19
vaccine:
https://www.thelancet.com/journals/langas/article/PIIS2468-1253(21)00197-7/
Management of cerebral and splanchnic vein thrombosis
associated with thrombocytopenia in subjects previously vaccinated
with Vaxzevria (AstraZeneca): position statement of the Italian
Society for the Study of Hemostasis and Thrombosis (SISET):
https://pubmed.ncbi.nlm.nih.gov/33871350/
Vaccine-induced immune immune thrombotic thrombocytopenia and
cerebral venous sinus thrombosis after vaccination with COVID-19; a
systematic review:
https://www.sciencedirect.com/science/article/pii/S0022510X21003014
Thrombosis with thrombocytopenia syndrome associated with
COVID-19 vaccines:
https://www.sciencedirect.com/science/article/abs/pii/S0735675721004381
Covid-19 vaccine-induced thrombosis and thrombocytopenia: a
commentary on an important and practical clinical dilemma:
https://www.sciencedirect.com/science/article/abs/pii/S0033062021000505
Thrombosis with thrombocytopenia syndrome associated with
COVID-19 viral vector vaccines:
https://www.sciencedirect.com/science/article/abs/pii/S0953620521001904
COVID-19 vaccine-induced immune-immune thrombotic
thrombocytopenia: an emerging cause of splanchnic vein thrombosis:
https://www.sciencedirect.com/science/article/pii/S1665268121000557
The roles of platelets in COVID-19-associated coagulopathy and
vaccine-induced immune thrombotic immune thrombocytopenia (covid):
https://www.sciencedirect.com/science/article/pii/S1050173821000967
Roots of autoimmunity of thrombotic events after COVID-19
vaccination:
https://www.sciencedirect.com/science/article/abs/pii/S1568997221002160
Cerebral venous sinus thrombosis after vaccination: the United
Kingdom experience:
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01788-8/fulltext
Thrombotic immune thrombocytopenia induced by SARS-CoV-2
vaccine: https://www.nejm.org/doi/full/10.1056/nejme2106315
Myocarditis after immunization with COVID-19 mRNA vaccines in
members of the US military. This article reports that in „23 male
patients, including 22 previously healthy military members,
myocarditis was identified within 4 days after receipt of the
vaccine”:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2781601
Thrombosis and thrombocytopenia after vaccination with ChAdOx1
nCoV-19:
https://www.nejm.org/doi/full/10.1056/NEJMoa2104882?query=recirc_curatedRelated_article
Association of myocarditis with the BNT162b2 messenger RNA
COVID-19 vaccine in a case series of children:
https://pubmed.ncbi.nlm.nih.gov/34374740/
Thrombotic thrombocytopenia after vaccination with ChAdOx1
nCov-19:
https://www.nejm.org/doi/full/10.1056/NEJMoa2104840?query=recirc_curatedRelated_article
Post-mortem findings in vaccine-induced thrombotic
thrombocytopenia (covid-19):
https://haematologica.org/article/view/haematol.2021.279075
Thrombocytopenia, including immune thrombocytopenia after
receiving COVID-19 mRNA vaccines reported to the Vaccine Adverse
Event Reporting System (VAERS):
https://www.sciencedirect.com/science/article/pii/S0264410X21005247
Acute symptomatic myocarditis in seven adolescents after
Pfizer-BioNTech COVID-19 vaccination:
https://pediatrics.aappublications.org/content/early/2021/06/04/peds.2021-052478
Aphasia seven days after the second dose of an mRNA-based
SARS-CoV-2 vaccine. Brain MRI revealed an intracerebral hemorrhage
(ICBH) in the left temporal lobe in a 52-year-old man.
https://www.sciencedirect.com/science/article/pii/S2589238X21000292#f0005
Comparison of vaccine-induced thrombotic episodes between
ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines:
https://www.sciencedirect.com/science/article/abs/pii/S0896841121000895
Hypothesis behind the very rare cases of thrombosis with
thrombocytopenia syndrome after SARS-CoV-2 vaccination:
https://www.sciencedirect.com/science/article/abs/pii/S0049384821003315
Blood clots and bleeding episodes after BNT162b2 and ChAdOx1
nCoV-19 vaccination: analysis of European data:
https://www.sciencedirect.com/science/article/pii/S0896841121000937
Cerebral venous thrombosis after BNT162b2 mRNA SARS-CoV-2
vaccine:
https://www.sciencedirect.com/science/article/abs/pii/S1052305721003098
Primary adrenal insufficiency associated with thrombotic
immune thrombocytopenia induced by the Oxford-AstraZeneca ChAdOx1
nCoV-19 vaccine (VITT):
https://www.sciencedirect.com/science/article/pii/S0953620521002363
Myocarditis and pericarditis after vaccination with COVID-19
mRNA: practical considerations for care providers:
https://www.sciencedirect.com/science/article/pii/S0828282X21006243
„Portal vein thrombosis occurring after the first dose of
SARS-CoV-2 mRNA vaccine in a patient with antiphospholipid syndrome”:
https://www.sciencedirect.com/science/article/pii/S2666572721000389
Early results of bivalirudin treatment for thrombotic
thrombocytopenia and cerebral venous sinus thrombosis after
vaccination with Ad26.COV2.S:
https://www.sciencedirect.com/science/article/pii/S0196064421003425
Myocarditis, pericarditis and cardiomyopathy after COVID-19
vaccination:
https://www.sciencedirect.com/science/article/pii/S1443950621011562
Mechanisms of immunothrombosis in vaccine-induced thrombotic
thrombocytopenia (VITT) compared to natural SARS-CoV-2 infection:
https://www.sciencedirect.com/science/article/abs/pii/S0896841121000706
Prothrombotic immune thrombocytopenia after COVID-19
vaccination:
https://www.sciencedirect.com/science/article/pii/S0006497121009411
Vaccine-induced thrombotic thrombocytopenia: the dark chapter
of a success story:
https://www.sciencedirect.com/science/article/pii/S2589936821000256
Cerebral venous sinus thrombosis negative for anti-PF4
antibody without thrombocytopenia after immunization with COVID-19
vaccine in a non-comorbid elderly Indian male treated with
conventional heparin-warfarin based anticoagulation:
https://www.sciencedirect.com/science/article/pii/S1871402121002046
Thrombosis after COVID-19 vaccination: possible link to ACE
pathways:
https://www.sciencedirect.com/science/article/pii/S0049384821004369
Cerebral venous sinus thrombosis in the U.S. population after
SARS-CoV-2 vaccination with adenovirus and after COVID-19:
https://www.sciencedirect.com/science/article/pii/S0735109721051949
A rare case of a middle-aged Asian male with cerebral venous
thrombosis after AstraZeneca COVID-19 vaccination:
https://www.sciencedirect.com/science/article/pii/S0735675721005714
Cerebral venous sinus thrombosis and thrombocytopenia after
COVID-19 vaccination: report of two cases in the United Kingdom:
https://www.sciencedirect.com/science/article/abs/pii/S088915912100163X
Immune thrombocytopenic purpura after vaccination with
COVID-19 vaccine (ChAdOx1 nCov-19):
https://www.sciencedirect.com/science/article/abs/pii/S0006497121013963.
Antiphospholipid antibodies and risk of thrombophilia after
COVID-19 vaccination: the straw that breaks the camel’s back?:
https://docs.google.com/document/d/1XzajasO8VMMnC3CdxSBKks1o7kiOLXFQ
Vaccine-induced thrombotic thrombocytopenia, a rare but severe
case of friendly fire in the battle against the COVID-19 pandemic:
What pathogenesis?:
https://www.sciencedirect.com/science/article/pii/S0953620521002314
Diagnostic-therapeutic recommendations of the ad-hoc FACME
expert working group on the management of cerebral venous thrombosis
related to COVID-19 vaccination:
https://www.sciencedirect.com/science/article/pii/S0213485321000839
Thrombocytopenia and intracranial venous sinus thrombosis
after exposure to the „AstraZeneca COVID-19 vaccine”:
https://pubmed.ncbi.nlm.nih.gov/33918932/
Thrombocytopenia following Pfizer and Moderna SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/33606296/
Severe and refractory immune thrombocytopenia occurring after
SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33854395/
Purpuric rash and thrombocytopenia after mRNA-1273 (Modern)
COVID-19 vaccine:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7996471/
COVID-19 vaccination: information on the occurrence of
arterial and venous thrombosis using data from VigiBase:
https://pubmed.ncbi.nlm.nih.gov/33863748/
Cerebral venous thrombosis associated with the covid-19
vaccine in Germany:
https://onlinelibrary.wiley.com/doi/10.1002/ana.26172
Cerebral venous thrombosis following BNT162b2 mRNA vaccination
of BNT162b2 against SARS-CoV-2: a black swan event:
https://pubmed.ncbi.nlm.nih.gov/34133027/
The importance of recognizing cerebral venous thrombosis
following anti-COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34001390/
Thrombosis with thrombocytopenia after messenger RNA vaccine
-1273: https://pubmed.ncbi.nlm.nih.gov/34181446/
Blood clots and bleeding after BNT162b2 and ChAdOx1 nCoV-19
vaccination: an analysis of European data:
https://pubmed.ncbi.nlm.nih.gov/34174723/
First dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and
thrombocytopenic, thromboembolic, and hemorrhagic events in Scotland:
https://www.nature.com/articles/s41591-021-01408-4
Exacerbation of immune thrombocytopenia after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34075578/
First report of a de novo iTTP episode associated with a
COVID-19 mRNA-based anti-COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34105244/
PF4 immunoassays in vaccine-induced thrombotic
thrombocytopenia: https://www.nejm.org/doi/full/10.1056/NEJMc2106383
Antibody epitopes in vaccine-induced immune immune thrombotic
thrombocytopenia: https://www.nature.com/articles/s41586-021-03744-4
Myocarditis with COVID-19 mRNA vaccines:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.121.056135
Myocarditis and pericarditis after COVID-19 vaccination:
https://jamanetwork.com/journals/jama/fullarticle/2782900
Myocarditis temporally associated with COVID-19 vaccination:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.121.055891.
COVID-19 Vaccination Associated with Myocarditis in
Adolescents:
https://pediatrics.aappublications.org/content/pediatrics/early/2021/08/12/peds.2021-053427.full.pdf
Acute myocarditis after administration of BNT162b2 vaccine
against COVID-19: https://pubmed.ncbi.nlm.nih.gov/33994339/
Temporal association between COVID-19 vaccine Ad26.COV2.S and
acute myocarditis: case report and review of the literature:
https://www.sciencedirect.com/science/article/pii/S1553838921005789
COVID-19 vaccine-induced myocarditis: a case report with
review of the literature:
https://www.sciencedirect.com/science/article/pii/S1871402121002253
Potential association between COVID-19 vaccine and
myocarditis: clinical and CMR findings:
https://www.sciencedirect.com/science/article/pii/S1936878X2100485X
Recurrence of acute myocarditis temporally associated with
receipt of coronavirus mRNA disease vaccine 2019 (COVID-19) in a male
adolescent:
https://www.sciencedirect.com/science/article/pii/S002234762100617X
Fulminant myocarditis and systemic hyper inflammation
temporally associated with BNT162b2 COVID-19 mRNA vaccination in two
patients:
https://www.sciencedirect.com/science/article/pii/S0167527321012286.
Acute myocarditis after administration of BNT162b2 vaccine:
https://www.sciencedirect.com/science/article/pii/S2214250921001530
Lymphohistocytic myocarditis after vaccination with COVID-19
Ad26.COV2.S viral vector:
https://www.sciencedirect.com/science/article/pii/S2352906721001573
Myocarditis following vaccination with BNT162b2 in a healthy
male:
https://www.sciencedirect.com/science/article/pii/S0735675721005362
Acute myocarditis after Comirnaty (Pfizer) vaccination in a
healthy male with previous SARS-CoV-2 infection:
https://www.sciencedirect.com/science/article/pii/S1930043321005549
Myopericarditis after Pfizer mRNA COVID-19 vaccination in
adolescents:
https://www.sciencedirect.com/science/article/pii/S002234762100665X
Pericarditis after administration of BNT162b2 mRNA COVID-19
mRNA vaccine:
https://www.sciencedirect.com/science/article/pii/S1885585721002218
Acute myocarditis after vaccination with SARS-CoV-2 mRNA-1273
mRNA:
https://www.sciencedirect.com/science/article/pii/S2589790X21001931
Temporal relationship between the second dose of BNT162b2 mRNA
Covid-19 vaccine and cardiac involvement in a patient with previous
SARS-COV-2 infection:
https://www.sciencedirect.com/science/article/pii/S2352906721000622
Myopericarditis after vaccination with COVID-19 mRNA in
adolescents 12 to 18 years of age:
https://www.sciencedirect.com/science/article/pii/S0022347621007368
Acute myocarditis after SARS-CoV-2 vaccination in a
24-year-old man:
https://www.sciencedirect.com/science/article/pii/S0870255121003243
Important information on myopericarditis after vaccination
with Pfizer COVID-19 mRNA in adolescents:
https://www.sciencedirect.com/science/article/pii/S0022347621007496
A series of patients with myocarditis after vaccination
against SARS-CoV-2 with mRNA-1279 and BNT162b2:
https://www.sciencedirect.com/science/article/pii/S1936878X21004861
Takotsubo cardiomyopathy after vaccination with mRNA COVID-19:
https://www.sciencedirect.com/science/article/pii/S1443950621011331
COVID-19 mRNA vaccination and myocarditis:
https://pubmed.ncbi.nlm.nih.gov/34268277/
COVID-19 vaccine and myocarditis:
https://pubmed.ncbi.nlm.nih.gov/34399967/
Epidemiology and clinical features of myocarditis/pericarditis
before the introduction of COVID-19 mRNA vaccine in Korean children:
a multicenter study
https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resourc
e/en/covidwho-1360706.
COVID-19 vaccines and myocarditis:
https://pubmed.ncbi.nlm.nih.gov/34246566/
Myocarditis and other cardiovascular complications of COVID-19
mRNA-based COVID-19 vaccines
https://www.cureus.com/articles/61030-myocarditis-and-other-cardiovascular-comp
lications-of-the-mrna-based-covid-19-vaccines
https://www.cureus.com/articles/61030-myocarditis-and-other-cardiovascular-complications-of-the-mrna-based-covid-19-vaccines
Myocarditis, pericarditis, and cardiomyopathy after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34340927/
Myocarditis with covid-19 mRNA vaccines:
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.056135
Association of myocarditis with COVID-19 mRNA vaccine in
children:
https://media.jamanetwork.com/news-item/association-of-myocarditis-with-mrna-co
vid-19-vaccine-in-children/
Association of myocarditis with COVID-19 messenger RNA vaccine
BNT162b2 in a case series of children:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2783052
Myocarditis after immunization with COVID-19 mRNA vaccines in
members of the U.S. military:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2781601%5C
Myocarditis occurring after immunization with COVID-19
mRNA-based COVID-19 vaccines:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2781600
Myocarditis following immunization with Covid-19 mRNA:
https://www.nejm.org/doi/full/10.1056/NEJMc2109975
Patients with acute myocarditis after vaccination withCOVID-19
mRNA:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2781602
Myocarditis associated with vaccination with COVID-19 mRNA:
https://pubs.rsna.org/doi/10.1148/radiol.2021211430
Symptomatic Acute Myocarditis in 7 Adolescents after
Pfizer-BioNTech COVID-19 Vaccination:
https://pediatrics.aappublications.org/content/148/3/e2021052478
Cardiovascular magnetic resonance imaging findings in young
adult patients with acute myocarditis after COVID-19 mRNA
vaccination: a case series:
https://jcmr-online.biomedcentral.com/articles/10.1186/s12968-021-00795-4
Clinical Guidance for Young People with Myocarditis and
Pericarditis after Vaccination with COVID-19 mRNA:
https://www.cps.ca/en/documents/position/clinical-guidance-for-youth-with-myocarditis-and-pericarditis
Cardiac imaging of acute myocarditis after vaccination with
COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34402228/
Case report: acute myocarditis after second dose of mRNA-1273
SARS-CoV-2 mRNA vaccine:
https://academic.oup.com/ehjcr/article/5/8/ytab319/6339567
Myocarditis / pericarditis associated with COVID-19 vaccine:
https://science.gc.ca/eic/site/063.nsf/eng/h_98291.html
Transient cardiac injury in adolescents receiving the BNT162b2
mRNA COVID-19 vaccine:
https://journals.lww.com/pidj/Abstract/9000/Transient_Cardiac_Injury_in_Adolesce
nts_Receiving.95800.aspx
Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19
vaccine:
https://academic.oup.com/jpids/advance-article/doi/10.1093/jpids/piab060/6329543
The new COVID-19 mRNA vaccine platform and myocarditis: clues
to the possible underlying mechanism:
https://pubmed.ncbi.nlm.nih.gov/34312010/
Acute myocardial injury after COVID-19 vaccination: a case
report and review of current evidence from the Vaccine Adverse Event
Reporting System database: https://pubmed.ncbi.nlm.nih.gov/34219532/
Be alert to the risk of adverse cardiovascular events after
COVID-19 vaccination:
https://www.xiahepublishing.com/m/2472-0712/ERHM-2021-00033
Myocarditis associated with COVID-19 vaccination:
echocardiographic, cardiac tomography, and magnetic resonance imaging
findings:
https://www.ahajournals.org/doi/10.1161/CIRCIMAGING.121.013236
In-depth evaluation of a case of presumed myocarditis after
the second dose of COVID-19 mRNA vaccine:
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.056038
Occurrence of acute infarct-like myocarditis after COVID-19
vaccination: just an accidental coincidence or rather a
vaccination-associated autoimmune myocarditis?:
https://pubmed.ncbi.nlm.nih.gov/34333695/
Recurrence of acute myocarditis temporally associated with
receipt of coronavirus mRNA disease vaccine 2019 (COVID-19) in a male
adolescent: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8216855/
Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced
reaction?: https://pubmed.ncbi.nlm.nih.gov/34118375/
Self-limited myocarditis presenting with chest pain and
ST-segment elevation in adolescents after vaccination with the
BNT162b2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34180390/
Myopericarditis in a previously healthy adolescent male after
COVID-19 vaccination: Case report:
https://pubmed.ncbi.nlm.nih.gov/34133825/
Biopsy-proven lymphocytic myocarditis after first COVID-19
mRNA vaccination in a 40-year-old man: case report:
https://pubmed.ncbi.nlm.nih.gov/34487236/
Insights from a murine model of COVID-19 mRNA vaccine-induced
myopericarditis: could accidental intravenous injection of a vaccine
induce myopericarditis
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab741/6359059
Unusual presentation of acute perimyocarditis after modern
SARS-COV-2 mRNA-1237 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34447639/
Perimyocarditis after the first dose of mRNA-1273 SARS-CoV-2
(Modern) mRNA-1273 vaccine in a young healthy male: case report:
https://bmccardiovascdisord.biomedcentral.com/articles/10.1186/s12872-021-02183
Acute myocarditis after the second dose of SARS-CoV-2 vaccine:
serendipity or causal relationship:
https://pubmed.ncbi.nlm.nih.gov/34236331/
Rhabdomyolysis and fasciitis induced by the COVID-19 mRNA
vaccine: https://pubmed.ncbi.nlm.nih.gov/34435250/
COVID-19 vaccine-induced rhabdomyolysis: case report with
literature review: https://pubmed.ncbi.nlm.nih.gov/34186348/.
GM1 ganglioside antibody and COVID-19-related Guillain Barre
syndrome: case report, systemic review, and implications for vaccine
development:
https://www.sciencedirect.com/science/article/pii/S2666354621000065
Guillain-Barré syndrome after AstraZeneca COVID-19
vaccination: causal or casual association:
https://www.sciencedirect.com/science/article/pii/S0303846721004169
Sensory Guillain-Barré syndrome after ChAdOx1 nCov-19
vaccine: report of two cases and review of the literature:
https://www.sciencedirect.com/science/article/pii/S0165572821002186
Guillain-Barré syndrome after the first dose of SARS-CoV-2
vaccine: a temporary occurrence, not a causal association:
https://www.sciencedirect.com/science/article/pii/S2214250921000998.
Guillain-Barré syndrome presenting as facial diplegia after
vaccination with COVID-19: a case report:
https://www.sciencedirect.com/science/article/pii/S0736467921006442
Guillain-Barré syndrome after the first injection of ChAdOx1
nCoV-19 vaccine: first report:
https://www.sciencedirect.com/science/article/pii/S0035378721005853.
SARS-CoV-2 vaccines are not safe for those with Guillain-Barre
syndrome following vaccination:
https://www.sciencedirect.com/science/article/pii/S2049080121005343
Acute hyperactive encephalopathy following COVID-19
vaccination with dramatic response to methylprednisolone: a case
report:
https://www.sciencedirect.com/science/article/pii/S2049080121007536
Facial nerve palsy following administration of COVID-19 mRNA
vaccines: analysis of self-report database:
https://www.sciencedirect.com/science/article/pii/S1201971221007049
Neurological symptoms and neuroimaging alterations related to
COVID-19 vaccine: cause or coincidence:
https://www.sciencedirect.com/science/article/pii/S0899707121003557.
New-onset refractory status epilepticus after ChAdOx1 nCoV-19
vaccination:
https://www.sciencedirect.com/science/article/pii/S0165572821001569
Acute myelitis and ChAdOx1 nCoV-19 vaccine: coincidental or
causal association:
https://www.sciencedirect.com/science/article/pii/S0165572821002137
Bell’s palsy and SARS-CoV-2 vaccines: an unfolding story:
https://www.sciencedirect.com/science/article/pii/S1473309921002735
Bell’s palsy after the second dose of the Pfizer COVID-19
vaccine in a patient with a history of recurrent Bell’s palsy:
https://www.sciencedirect.com/science/article/pii/S266635462100020X
Acute-onset central serous retinopathy after immunization with
COVID-19 mRNA vaccine:.
https://www.sciencedirect.com/science/article/pii/S2451993621001456.
Bell’s palsy after COVID-19 vaccination: case report:
https://www.sciencedirect.com/science/article/pii/S217358082100122X.
An academic hospital experience assessing the risk of COVID-19
mRNA vaccine using patient’s allergy history:
https://www.sciencedirect.com/science/article/pii/S2213219821007972
COVID-19 vaccine-induced axillary and pectoral lymphadenopathy
in PET:
https://www.sciencedirect.com/science/article/pii/S1930043321002612
ANCA-associated vasculitis after Pfizer-BioNTech COVID-19
vaccine:
https://www.sciencedirect.com/science/article/pii/S0272638621007423
Late cutaneous reactions after administration of COVID-19 mRNA
vaccines:
https://www.sciencedirect.com/science/article/pii/S2213219821007996
COVID-19 vaccine-induced rhabdomyolysis: case report with
review of the literature:
https://www.sciencedirect.com/science/article/pii/S1871402121001880
Clinical and pathologic correlates of skin reactions to
COVID-19 vaccine, including V-REPP: a registry-based study:
https://www.sciencedirect.com/science/article/pii/S0190962221024427
Thrombosis with thrombocytopenia syndrome associated with
COVID-19 vaccines:.
https://www.sciencedirect.com/science/article/abs/pii/S0735675721004381.
COVID-19 vaccine-associated anaphylaxis: a statement from the
Anaphylaxis Committee of the World Allergy Organization:.
https://www.sciencedirect.com/science/article/pii/S1939455121000119.
Cerebral venous sinus thrombosis negative for anti-PF4
antibody without thrombocytopenia after immunization with COVID-19
vaccine in an elderly, non-comorbid Indian male treated with
conventional heparin-warfarin-based anticoagulation:.
https://www.sciencedirect.com/science/article/pii/S1871402121002046.
Acute myocarditis after administration of BNT162b2 vaccine
against COVID-19:.
https://www.sciencedirect.com/science/article/abs/pii/S188558572100133X
Blood clots and bleeding after BNT162b2 and ChAdOx1 nCoV-19
vaccine: an analysis of European data:.
https://www.sciencedirect.com/science/article/pii/S0896841121000937.
immune thrombocytopenia associated with Pfizer-BioNTech’s
COVID-19 BNT162b2 mRNA vaccine:.
https://www.sciencedirect.com/science/article/pii/S2214250921002018.
Bullous drug eruption after the second dose of COVID-19
mRNA-1273 (Moderna) vaccine: Case report:
https://www.sciencedirect.com/science/article/pii/S1876034121001878.
COVID-19 RNA-based vaccines and the risk of prion disease:
https://scivisionpub.com/pdfs/covid19rna-based-vaccines-and-the-risk-of-prion-dis
ease-1503.pdf
This study notes that 115 pregnant women lost their babies,
out of 827 who participated in a study on the safety of covid-19
vaccines: https://www.nejm.org/doi/full/10.1056/NEJMoa2104983.
Process-related impurities in the ChAdOx1 nCov-19 vaccine:
https://www.researchsquare.com/article/rs-477964/v1
COVID-19 mRNA vaccine causing CNS inflammation: a case series:
https://link.springer.com/article/10.1007/s00415-021-10780-7
Allergic reactions, including anaphylaxis, after receiving the
first dose of the Pfizer-BioNTech COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33475702/
Allergic reactions to the first COVID-19 vaccine: a potential
role of polyethylene glycol:
https://pubmed.ncbi.nlm.nih.gov/33320974/
Pfizer Vaccine Raises Allergy Concerns:
https://pubmed.ncbi.nlm.nih.gov/33384356/
Allergic reactions, including anaphylaxis, after receiving the
first dose of Pfizer-BioNTech COVID-19 vaccine – United States,
December 14-23, 2020: https://pubmed.ncbi.nlm.nih.gov/33444297/
Allergic reactions, including anaphylaxis, after receiving
first dose of Modern COVID-19 vaccine – United States, December 21,
2020-January 10, 2021: https://pubmed.ncbi.nlm.nih.gov/33507892/
Reports of anaphylaxis after coronavirus disease vaccination
2019, South Korea, February 26-April 30, 2021:
https://pubmed.ncbi.nlm.nih.gov/34414880/
Reports of anaphylaxis after receiving COVID-19 mRNA vaccines
in the U.S.-Dec 14, 2020-Jan 18, 2021:
https://pubmed.ncbi.nlm.nih.gov/33576785/
Immunization practices and risk of anaphylaxis: a current,
comprehensive update of COVID-19 vaccination data:
https://pubmed.ncbi.nlm.nih.gov/34269740/
Relationship between pre-existing allergies and anaphylactic
reactions following administration of COVID-19 mRNA vaccine:
https://pubmed.ncbi.nlm.nih.gov/34215453/
Anaphylaxis Associated with COVID-19 mRNA Vaccines: Approach
to Allergy Research: https://pubmed.ncbi.nlm.nih.gov/33932618/
Severe Allergic Reactions after COVID-19 Vaccination with the
Pfizer / BioNTech Vaccine in Great Britain and the USA: Position
Statement of the German Allergy Societies: German Medical Association
of Allergologists (AeDA), German Society for Allergology and Clinical
Immunology (DGAKI) and Society for Pediatric Allergology and
Environmental Medicine (GPA):
https://pubmed.ncbi.nlm.nih.gov/33643776/
Allergic reactions and anaphylaxis to LNP-based COVID-19
vaccines: https://pubmed.ncbi.nlm.nih.gov/33571463/
Reported orofacial adverse effects from COVID-19 vaccines: the
known and the unknown: https://pubmed.ncbi.nlm.nih.gov/33527524/
Cutaneous adverse effects of available COVID-19 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34518015/
Cumulative adverse event report of anaphylaxis following
injections of COVID-19 mRNA vaccine (Pfizer-BioNTech) in Japan: the
first month report: https://pubmed.ncbi.nlm.nih.gov/34347278/
COVID-19 vaccines increase the risk of anaphylaxis:
https://pubmed.ncbi.nlm.nih.gov/33685103/
Biphasic anaphylaxis after exposure to the first dose of the
Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34050949/
Allergenic components of the mRNA-1273 vaccine for COVID-19:
possible involvement of polyethylene glycol and IgG-mediated
complement activation: https://pubmed.ncbi.nlm.nih.gov/33657648/
Polyethylene glycol (PEG) is a cause of anaphylaxis to Pfizer
/ BioNTech mRNA COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33825239/
Acute allergic reactions to COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/33683290/
Polyethylene glycole allergy of the SARS CoV2 vaccine
recipient: case report of a young adult recipient and management of
future exposure to SARS-CoV2:
https://pubmed.ncbi.nlm.nih.gov/33919151/
Elevated rates of anaphylaxis after vaccination with Pfizer
BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare
workers; a secondary analysis of initial post-approval safety data:
https://pubmed.ncbi.nlm.nih.gov/34128049/
Allergic reactions and adverse events associated with
administration of mRNA-based vaccines. A health system experience:
https://pubmed.ncbi.nlm.nih.gov/34474708/
Allergic reactions to COVID-19 vaccines: statement of the
Belgian Society of Allergy and Clinical Immunology (BelSACI):
https://www.tandfonline.com/doi/abs/10.1080/17843286.2021.1909447
.IgE-mediated allergy to polyethylene glycol (PEG) as a cause
of anaphylaxis to COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/34318537/
Allergic reactions after COVID-19 vaccination: putting the
risk in perspective: https://pubmed.ncbi.nlm.nih.gov/34463751/
Anaphylactic reactions to COVID-19 mRNA vaccines: a call for
further studies: https://pubmed.ncbi.nlm.nih.gov/33846043/ 188.
Risk of severe allergic reactions to COVID-19 vaccines among
patients with allergic skin disease: practical recommendations. An
ETFAD position statement with external experts:
https://pubmed.ncbi.nlm.nih.gov/33752263/
COVID-19 vaccine and death: causality algorithm according to
the WHO eligibility diagnosis:
https://pubmed.ncbi.nlm.nih.gov/34073536/
Fatal brain hemorrhage after COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33928772/
A case series of skin reactions to COVID-19 vaccine in the
Department of Dermatology at Loma Linda University:
https://pubmed.ncbi.nlm.nih.gov/34423106/
Skin reactions reported after Moderna and Pfizer’s COVID-19
vaccination: a study based on a registry of 414 cases:
https://pubmed.ncbi.nlm.nih.gov/33838206/
Clinical and pathologic correlates of skin reactions to
COVID-19 vaccine, including V-REPP: a registry-based study:
https://pubmed.ncbi.nlm.nih.gov/34517079/
Skin reactions after vaccination against SARS-COV-2: a
nationwide Spanish cross-sectional study of 405 cases:
https://pubmed.ncbi.nlm.nih.gov/34254291/
Varicella zoster virus and herpes simplex virus reactivation
after vaccination with COVID-19: review of 40 cases in an
international dermatologic registry:
https://pubmed.ncbi.nlm.nih.gov/34487581/
Immune thrombosis and thrombocytopenia (VITT) associated with
the COVID-19 vaccine: diagnostic and therapeutic recommendations for
a new syndrome: https://pubmed.ncbi.nlm.nih.gov/33987882/
Laboratory testing for suspicion of COVID-19 vaccine-induced
thrombotic (immune) thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34138513/
Intracerebral hemorrhage due to thrombosis with
thrombocytopenia syndrome after COVID-19 vaccination: the first fatal
case in Korea: https://pubmed.ncbi.nlm.nih.gov/34402235/
Risk of thrombocytopenia and thromboembolism after covid-19
vaccination and positive SARS-CoV-2 tests: self-controlled case
series study: https://pubmed.ncbi.nlm.nih.gov/34446426/
Vaccine-induced immune thrombotic thrombocytopenia and
cerebral venous sinus thrombosis after covid-19 vaccination; a
systematic review: https://pubmed.ncbi.nlm.nih.gov/34365148/.
Nerve and muscle adverse events after vaccination with
COVID-19: a systematic review and meta-analysis of clinical trials:
https://pubmed.ncbi.nlm.nih.gov/34452064/.
A rare case of cerebral venous thrombosis and disseminated
intravascular coagulation temporally associated with administration
of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33917902/
Primary adrenal insufficiency associated with thrombotic
immune thrombocytopenia induced by Oxford-AstraZeneca ChAdOx1 nCoV-19
vaccine (VITT): https://pubmed.ncbi.nlm.nih.gov/34256983/
Acute cerebral venous thrombosis and pulmonary artery embolism
associated with the COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34247246/.
Thromboaspiration infusion and fibrinolysis for
portomesenteric thrombosis after administration of AstraZeneca
COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34132839/
59-year-old woman with extensive deep venous thrombosis and
pulmonary thromboembolism 7 days after a first dose of
Pfizer-BioNTech BNT162b2 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34117206/
Cerebral venous thrombosis and vaccine-induced
thrombocytopenia.a. Oxford-AstraZeneca COVID-19: a missed opportunity
for a rapid return on experience:
https://pubmed.ncbi.nlm.nih.gov/34033927/
Myocarditis and other cardiovascular complications of
mRNA-based COVID-19 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34277198/
Pericarditis after administration of COVID-19 mRNA BNT162b2
vaccine: https://pubmed.ncbi.nlm.nih.gov/34364831/
Unusual presentation of acute pericarditis after vaccination
against SARS-COV-2 mRNA-1237 Modern:
https://pubmed.ncbi.nlm.nih.gov/34447639/
Case report: acute myocarditis after second dose of SARS-CoV-2
mRNA-1273 vaccine mRNA-1273:
https://pubmed.ncbi.nlm.nih.gov/34514306/
Immune-mediated disease outbreaks or recent-onset disease in
27 subjects after mRNA/DNA vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/33946748/
Insights from a murine model of myopericarditis induced by
COVID-19 mRNA vaccine: could accidental intravenous injection of a
vaccine induce myopericarditis:
https://pubmed.ncbi.nlm.nih.gov/34453510/
Immune thrombocytopenia in a 22-year-old post Covid-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/33476455/
propylthiouracil-induced neutrophil anti-cytoplasmic
antibody-associated vasculitis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34451967/
Secondary immune thrombocytopenia (ITP) associated with
ChAdOx1 Covid-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34377889/
Thrombosis with thrombocytopenia syndrome (TTS) following
AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination:
risk-benefit analysis for persons <60 years in Australia:
https://pubmed.ncbi.nlm.nih.gov/34272095/
COVID-19 vaccination association and facial nerve palsy: A
case-control study: https://pubmed.ncbi.nlm.nih.gov/34165512/
The association between COVID-19 vaccination and Bell’s
palsy: https://pubmed.ncbi.nlm.nih.gov/34411533/
Bell’s palsy after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33611630/
Acute transverse myelitis (ATM): clinical review of 43
patients with COVID-19-associated ATM and 3 serious adverse events of
post-vaccination ATM with ChAdOx1 nCoV-19 vaccine (AZD1222):
https://pubmed.ncbi.nlm.nih.gov/33981305/
Bell’s palsy after 24 hours of mRNA-1273 SARS-CoV-2
mRNA-1273 vaccine: https://pubmed.ncbi.nlm.nih.gov/34336436/
Sequential contralateral facial nerve palsy after first and
second doses of COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34281950/.
Transverse myelitis induced by SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34458035/
Peripheral facial nerve palsy after vaccination with BNT162b2
(COVID-19): https://pubmed.ncbi.nlm.nih.gov/33734623/
Acute abducens nerve palsy after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34044114/.
Facial nerve palsy after administration of COVID-19 mRNA
vaccines: analysis of self-report database:
https://pubmed.ncbi.nlm.nih.gov/34492394/
Transient oculomotor paralysis after administration of
RNA-1273 messenger vaccine for SARS-CoV-2 diplopia after COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34369471/
Bell’s palsy after Ad26.COV2.S COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34014316/
Bell’s palsy after COVID-19 vaccination: case report:
https://pubmed.ncbi.nlm.nih.gov/34330676/
A case of acute demyelinating polyradiculoneuropathy with
bilateral facial palsy following ChAdOx1 nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34272622/
Guillian Barré syndrome after vaccination with mRNA-1273
against COVID-19: https://pubmed.ncbi.nlm.nih.gov/34477091/
Acute facial paralysis as a possible complication of
SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/33975372/.
Bell’s palsy after COVID-19 vaccination with high antibody
response in CSF: https://pubmed.ncbi.nlm.nih.gov/34322761/.
Parsonage-Turner syndrome associated with SARS-CoV-2 or
SARS-CoV-2 vaccination. Comment on: „Neuralgic amyotrophy and
COVID-19 infection: 2 cases of accessory spinal nerve palsy” by
Coll et al. Articular Spine 2021; 88: 10519:
https://pubmed.ncbi.nlm.nih.gov/34139321/.
Bell’s palsy after a single dose of vaccine mRNA.
SARS-CoV-2: case report: https://pubmed.ncbi.nlm.nih.gov/34032902/.
Autoimmune hepatitis developing after coronavirus disease
vaccine 2019 (COVID-19): causality or victim?:
https://pubmed.ncbi.nlm.nih.gov/33862041/
Autoimmune hepatitis triggered by vaccination against
SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34332438/
Acute autoimmune-like hepatitis with atypical
antimitochondrial antibody after vaccination with COVID-19 mRNA: a
new clinical entity: https://pubmed.ncbi.nlm.nih.gov/34293683/.
Autoimmune hepatitis after COVID vaccine:
https://pubmed.ncbi.nlm.nih.gov/34225251/
A novel case of bifacial diplegia variant of Guillain-Barré
syndrome after vaccination with Janssen COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34449715/
Comparison of vaccine-induced thrombotic events between
ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines:
https://pubmed.ncbi.nlm.nih.gov/34139631/.
Bilateral superior ophthalmic vein thrombosis, ischemic stroke
and immune thrombocytopenia after vaccination with ChAdOx1 nCoV-19:
https://pubmed.ncbi.nlm.nih.gov/33864750/
Diagnosis and treatment of cerebral venous sinus thrombosis
with vaccine-induced immune-immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/33914590/
Venous sinus thrombosis after vaccination with ChAdOx1
nCov-19: https://pubmed.ncbi.nlm.nih.gov/34420802/
Cerebral venous sinus thrombosis following vaccination against
SARS-CoV-2: an analysis of cases reported to the European Medicines
Agency: https://pubmed.ncbi.nlm.nih.gov/34293217/
Risk of thrombocytopenia and thromboembolism after covid-19
vaccination and positive SARS-CoV-2 tests: self-controlled case
series study: https://pubmed.ncbi.nlm.nih.gov/34446426/
Blood clots and bleeding after BNT162b2 and ChAdOx1 nCoV-19
vaccination: an analysis of European data:
https://pubmed.ncbi.nlm.nih.gov/34174723/
Arterial events, venous thromboembolism, thrombocytopenia and
bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in
Denmark and Norway: population-based cohort study:
https://pubmed.ncbi.nlm.nih.gov/33952445/
First dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and
thrombocytopenic, thromboembolic and hemorrhagic events in Scotland:
https://pubmed.ncbi.nlm.nih.gov/34108714/
Cerebral venous thrombosis associated with COVID-19 vaccine in
Germany: https://pubmed.ncbi.nlm.nih.gov/34288044/
Malignant cerebral infarction after vaccination with ChAdOx1
nCov-19: a catastrophic variant of vaccine-induced immune-mediated
thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34341358/
celiac artery and splenic artery thrombosis complicated by
splenic infarction 7 days after the first dose of Oxford vaccine,
causal relationship or coincidence:
https://pubmed.ncbi.nlm.nih.gov/34261633/.
Primary adrenal insufficiency associated with
Oxford-AstraZeneca ChAdOx1 nCoV-19 (VITT) vaccine-induced immune
thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34256983/
Thrombocytopenia after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34332437/.
Cerebral venous sinus thrombosis associated with
thrombocytopenia after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33845870/.
Thrombosis with thrombocytopenia syndrome after COVID-19
immunization: https://pubmed.ncbi.nlm.nih.gov/34236343/
Acute myocardial infarction within 24 hours after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34364657/.
Bilateral acute macular neuroretinopathy after SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34287612/
central venous sinus thrombosis with subarachnoid hemorrhage
after COVID-19 mRNA vaccination: are these reports merely
coincidental: https://pubmed.ncbi.nlm.nih.gov/34478433/
Intracerebral hemorrhage due to thrombosis with
thrombocytopenia syndrome after COVID-19 vaccination: the first fatal
case in Korea: https://pubmed.ncbi.nlm.nih.gov/34402235/
Cerebral venous sinus thrombosis negative for anti-PF4
antibody without thrombocytopenia after immunization with COVID-19
vaccine in a non-comorbid elderly Indian male treated with
conventional heparin-warfarin-based anticoagulation:
https://pubmed.ncbi.nlm.nih.gov/34186376/
Cerebral venous sinus thrombosis 2 weeks after first dose of
SARS-CoV-2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34101024/
A case of multiple thrombocytopenia and thrombosis following
vaccination with ChAdOx1 nCoV-19 against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/34137813/
Vaccine-induced thrombotic thrombocytopenia: the elusive link
between thrombosis and adenovirus-based SARS-CoV-2 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34191218/
Acute ischemic stroke revealing immune thrombotic
thrombocytopenia induced by ChAdOx1 nCov-19 vaccine: impact on
recanalization strategy: https://pubmed.ncbi.nlm.nih.gov/34175640/
New-onset refractory status epilepticus after ChAdOx1 nCoV-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34153802/
Thrombosis with thrombocytopenia syndrome associated with
COVID-19 viral vector vaccines:
https://pubmed.ncbi.nlm.nih.gov/34092488/
Pulmonary embolism, transient ischemic attack, and
thrombocytopenia after Johnson & Johnson COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34261635/
Thromboaspiration infusion and fibrinolysis for
portomesenteric thrombosis after administration of the AstraZeneca
COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34132839/.
Spontaneous HIT syndrome: knee replacement, infection, and
parallels with vaccine-induced immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34144250/
Deep venous thrombosis (DVT) occurring shortly after second
dose of SARS-CoV-2 mRNA vaccine:
https://pubmed.ncbi.nlm.nih.gov/33687691/
Procoagulant antibody-mediated procoagulant platelets in
immune thrombotic thrombocytopenia associated with SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34011137/.
Vaccine-induced immune thrombotic thrombocytopenia causing a
severe form of cerebral venous thrombosis with high mortality rate: a
case series: https://pubmed.ncbi.nlm.nih.gov/34393988/.
Procoagulant microparticles: a possible link between
vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus
venous thrombosis: https://pubmed.ncbi.nlm.nih.gov/34129181/.
Atypical thrombosis associated with the vaccine VaxZevria®
(AstraZeneca): data from the French network of regional
pharmacovigilance centers: https://pubmed.ncbi.nlm.nih.gov/34083026/.
Acute cerebral venous thrombosis and pulmonary artery embolism
associated with the COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34247246/.
Vaccine-induced thrombosis and thrombocytopenia with bilateral
adrenal haemorrhage: https://pubmed.ncbi.nlm.nih.gov/34235757/.
Palmar digital vein thrombosis after Oxford-AstraZeneca
COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34473841/.
Cutaneous thrombosis associated with cutaneous necrosis
following Oxford-AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34189756/
Cerebral venous thrombosis following COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34045111/.
Lipschütz ulcers after AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34366434/.
Amyotrophic Neuralgia secondary to Vaxzevri vaccine
(AstraZeneca) COVID-19: https://pubmed.ncbi.nlm.nih.gov/34330677/
Thrombosis with thrombocytopenia after Messenger vaccine
RNA-1273: https://pubmed.ncbi.nlm.nih.gov/34181446/
Intracerebral hemorrhage twelve days after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34477089/
Thrombotic thrombocytopenia after vaccination with COVID-19:
in search of the underlying mechanism:
https://pubmed.ncbi.nlm.nih.gov/34071883/
Coronavirus (COVID-19) Vaccine-induced immune thrombotic
thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34033367/
Comparison of adverse drug reactions among four COVID-19
vaccines in Europe using the EudraVigilance database: Thrombosis in
unusual sites: https://pubmed.ncbi.nlm.nih.gov/34375510/
Immunoglobulin adjuvant for vaccine-induced immune thrombotic
thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34107198/
Severe vaccine-induced thrombotic thrombocytopenia following
vaccination with COVID-19: an autopsy case report and review of the
literature: https://pubmed.ncbi.nlm.nih.gov/34355379/.
A case of acute pulmonary embolism after immunization with
SARS-CoV-2 mRNA: https://pubmed.ncbi.nlm.nih.gov/34452028/
Neurosurgical considerations regarding decompressive
craniectomy for intracerebral hemorrhage after SARS-CoV-2 vaccination
in vaccine-induced thrombotic thrombocytopenia-VITT:
https://pubmed.ncbi.nlm.nih.gov/34202817/
Thrombosis and SARS-CoV-2 vaccines: vaccine-induced immune
thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34237213/.
Acquired thrombotic thrombocytopenic thrombocytopenic purpura:
a rare disease associated with the BNT162b2 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34105247/.
Immune complexes, innate immunity and NETosis in ChAdOx1
vaccine-induced thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34405870/.
Sensory Guillain-Barré syndrome following ChAdOx1 nCov-19
vaccine: report of two cases and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34416410/.
Vogt-Koyanagi-Harada syndrome after COVID-19 and ChAdOx1
nCoV-19 (AZD1222) vaccination:
https://pubmed.ncbi.nlm.nih.gov/34462013/.
Reactivation of Vogt-Koyanagi-Harada disease under control for
more than 6 years, after anti-SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34224024/.
Post-vaccinal encephalitis after ChAdOx1 nCov-19:
https://pubmed.ncbi.nlm.nih.gov/34324214/
Neurological symptoms and neuroimaging alterations related to
COVID-19 vaccine: cause or coincidence?:
https://pubmed.ncbi.nlm.nih.gov/34507266/
Fatal systemic capillary leak syndrome after SARS-COV-2
vaccination in a patient with multiple myeloma:
https://pubmed.ncbi.nlm.nih.gov/34459725/
Polyarthralgia and myalgia syndrome after vaccination with
ChAdOx1 nCOV-19: https://pubmed.ncbi.nlm.nih.gov/34463066/
Three cases of subacute thyroiditis after SARS-CoV-2
vaccination: post-vaccination ASIA syndrome:
https://pubmed.ncbi.nlm.nih.gov/34043800/.
Facial diplegia: a rare and atypical variant of Guillain-Barré
syndrome and the Ad26.COV2.S vaccine:
https://pubmed.ncbi.nlm.nih.gov/34447646/
Association between ChAdOx1 nCoV-19 vaccination and bleeding
episodes: large population-based cohort study:
https://pubmed.ncbi.nlm.nih.gov/34479760/.
fulminant myocarditis and systemic hyperinflammation
temporally associated with BNT162b2 COVID-19 mRNA vaccination in two
patients: https://pubmed.ncbi.nlm.nih.gov/34416319/.
Adverse effects reported after COVID-19 vaccination in a
tertiary care hospital, centered on cerebral venous sinus thrombosis
(CVST): https://pubmed.ncbi.nlm.nih.gov/34092166/
Induction and exacerbation of subacute cutaneous lupus
erythematosus erythematosus after mRNA- or adenoviral vector-based
SARS-CoV-2 vaccination: https://pubmed.ncbi.nlm.nih.gov/34291477/
Petechiae and peeling of fingers after immunization with
BTN162b2 messenger RNA (mRNA)-based COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34513435/
Hepatitis C virus reactivation after COVID-19 vaccination: a
case report: https://pubmed.ncbi.nlm.nih.gov/34512037/
Bilateral immune-mediated keratolysis after immunization with
SARS-CoV-2 recombinant viral vector vaccine:
https://pubmed.ncbi.nlm.nih.gov/34483273/.
Immune-mediated thrombocytopenic purpura after Pfizer-BioNTech
COVID-19 vaccine in an elderly woman:
https://pubmed.ncbi.nlm.nih.gov/34513446/
Platelet activation and modulation in thrombosis with
thrombocytopenia syndrome associated with the ChAdO × 1 nCov-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34474550/
Reactive arthritis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34033732/.
Two cases of Graves’ disease after SARS-CoV-2 vaccination:
an autoimmune / inflammatory syndrome induced by adjuvants:
https://pubmed.ncbi.nlm.nih.gov/33858208/
Acute relapse and impaired immunization after COVID-19
vaccination in a patient with multiple sclerosis treated with
rituximab: https://pubmed.ncbi.nlm.nih.gov/34015240/
Widespread fixed bullous drug eruption after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34482558/
COVID-19 mRNA vaccine causing CNS inflammation: a case series:
https://pubmed.ncbi.nlm.nih.gov/34480607/
Thymic hyperplasia after Covid-19 mRNA-based vaccination with
Covid-19: https://pubmed.ncbi.nlm.nih.gov/34462647/
Acute disseminated encephalomyelitis following vaccination
against SARS-CoV-2: https://pubmed.ncbi.nlm.nih.gov/34325334/
Tolosa-Hunt syndrome occurring after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34513398/
Systemic capillary extravasation syndrome following
vaccination with ChAdOx1 nCOV-19 (Oxford-AstraZeneca):
https://pubmed.ncbi.nlm.nih.gov/34362727/
Immune-mediated thrombocytopenia associated with Ad26.COV2.S
vaccine (Janssen; Johnson & Johnson):
https://pubmed.ncbi.nlm.nih.gov/34469919/.
Transient thrombocytopenia with glycoprotein-specific platelet
autoantibodies after vaccination with Ad26.COV2.S: case report:
https://pubmed.ncbi.nlm.nih.gov/34516272/.
Acute hyperactive encephalopathy following COVID-19
vaccination with dramatic response to methylprednisolone: case
report: https://pubmed.ncbi.nlm.nih.gov/34512961/
Transient cardiac injury in adolescents receiving the BNT162b2
mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34077949/
Autoimmune hepatitis developing after ChAdOx1 nCoV-19 vaccine
(Oxford-AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34171435/
Severe relapse of multiple sclerosis after COVID-19
vaccination: a case report: https://pubmed.ncbi.nlm.nih.gov/34447349/
Lymphohistocytic myocarditis after vaccination with the
COVID-19 viral vector Ad26.COV2.S:
https://pubmed.ncbi.nlm.nih.gov/34514078/
Hemophagocytic lymphohistiocytosis after vaccination with
ChAdOx1 nCov-19: https://pubmed.ncbi.nlm.nih.gov/34406660/.
IgA vasculitis in adult patient after vaccination with ChadOx1
nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34509658/
A case of leukocytoclastic vasculitis after vaccination with a
SARS-CoV2 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34196469/.
Onset / outbreak of psoriasis after Corona virus ChAdOx1
nCoV-19 vaccine (Oxford-AstraZeneca / Covishield): report of two
cases: https://pubmed.ncbi.nlm.nih.gov/34350668/
Hailey-Hailey disease exacerbation after SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34436620/
Supraclavicular lymphadenopathy after COVID-19 vaccination in
Korea: serial follow-up by ultrasonography:
https://pubmed.ncbi.nlm.nih.gov/34116295/.
COVID-19 vaccine, immune thrombotic thrombocytopenia,
jaundice, hyperviscosity: concern in cases with underlying hepatic
problems: https://pubmed.ncbi.nlm.nih.gov/34509271/.
Report of the International Cerebral Venous Thrombosis
Consortium on cerebral venous thrombosis after SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34462996/
Immune thrombocytopenia after vaccination during the COVID-19
pandemic: https://pubmed.ncbi.nlm.nih.gov/34435486/
COVID-19: lessons from the Norwegian tragedy should be taken
into account in planning for vaccine launch in less
developed/developing countries:
https://pubmed.ncbi.nlm.nih.gov/34435142/
Rituximab-induced acute lympholysis and pancytopenia following
vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34429981/
Exacerbation of plaque psoriasis after COVID-19 inactivated
mRNA and BNT162b2 vaccines: report of two cases:
https://pubmed.ncbi.nlm.nih.gov/34427024/
Vaccine-induced interstitial lung disease: a rare reaction to
COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34510014/.
Vesiculobullous cutaneous reactions induced by COVID-19 mRNA
vaccine: report of four cases and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34236711/
Vaccine-induced thrombocytopenia with severe headache:
https://pubmed.ncbi.nlm.nih.gov/34525282/
Acute perimyocarditis after the first dose of COVID-19 mRNA
vaccine: https://pubmed.ncbi.nlm.nih.gov/34515024/
Rhabdomyolysis and fasciitis induced by COVID-19 mRNA vaccine:
https://pubmed.ncbi.nlm.nih.gov/34435250/.
Rare cutaneous adverse effects of COVID-19 vaccines: a case
series and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34363637/
Immune thrombocytopenia associated with the Pfizer-BioNTech
COVID-19 mRNA vaccine BNT162b2:
https://www.sciencedirect.com/science/article/pii/S2214250921002018
Secondary immune thrombocytopenia putatively attributable to
COVID-19 vaccination:
https://casereports.bmj.com/content/14/5/e242220.abstract.
Immune thrombocytopenia following Pfizer-BioNTech BNT162b2
mRNA COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34155844/
Newly diagnosed idiopathic thrombocytopenia after COVID-19
vaccine administration:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8176657/.
Idiopathic thrombocytopenic purpura and the Modern Covid-19
vaccine:
https://www.annemergmed.com/article/S0196-0644(21)00122-0/fulltext.
Thrombocytopenia after Pfizer and Moderna SARS vaccination –
CoV -2: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8014568/.
Immune thrombocytopenic purpura and acute liver injury after
COVID-19 vaccination:
https://casereports.bmj.com/content/14/7/e242678.
Collection of complement-mediated and autoimmune-mediated
hematologic conditions after SARS-CoV-2 vaccination:
https://ashpublications.org/bloodadvances/article/5/13/2794/476324/Autoimmune-and-complement-mediated-hematologic
Petechial rash associated with CoronaVac vaccination: first
report of cutaneous side effects before phase 3 results:
https://ejhp.bmj.com/content/early/2021/05/23/ejhpharm-2021-002794
COVID-19 vaccines induce severe hemolysis in paroxysmal
nocturnal hemoglobinuria:
https://ashpublications.org/blood/article/137/26/3670/475905/COVID-19-vaccines-induce-severe-hemolysis-in
Cerebral venous thrombosis associated with COVID-19 vaccine in
Germany: https://pubmed.ncbi.nlm.nih.gov/34288044/.
Cerebral venous sinus thrombosis after COVID-19 vaccination :
Neurological and radiological management:
https://pubmed.ncbi.nlm.nih.gov/34327553/.
Cerebral venous thrombosis and thrombocytopenia after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33878469/.
Cerebral venous sinus thrombosis and thrombocytopenia after
COVID-19 vaccination: report of two cases in the United Kingdom:
https://pubmed.ncbi.nlm.nih.gov/33857630/.
Cerebral venous thrombosis induced by SARS-CoV-2 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34090750/.
Carotid artery immune thrombosis induced by
adenovirus-vectored COVID-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34312301/.
Cerebral venous sinus thrombosis associated with
vaccine-induced thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34333995/
The roles of platelets in COVID-19-associated coagulopathy and
vaccine-induced immune-immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34455073/
Cerebral venous thrombosis after the BNT162b2 mRNA SARS-CoV-2
vaccine: https://pubmed.ncbi.nlm.nih.gov/34111775/.
Cerebral venous thrombosis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34045111/
Lethal cerebral venous sinus thrombosis after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33983464/
Cerebral venous sinus thrombosis in the U.S. population, After
SARS-CoV-2 vaccination with adenovirus and after COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34116145/
Cerebral venous thrombosis after COVID-19 vaccination: is the
risk of thrombosis increased by intravascular administration of the
vaccine: https://pubmed.ncbi.nlm.nih.gov/34286453/.
Central venous sinus thrombosis with subarachnoid hemorrhage
after COVID-19 mRNA vaccination: are these reports merely
coincidental: https://pubmed.ncbi.nlm.nih.gov/34478433/
Cerebral venous sinus thrombosis after ChAdOx1 nCov-19
vaccination with a misleading first brain MRI:
https://pubmed.ncbi.nlm.nih.gov/34244448/
Early results of bivalirudin treatment for thrombotic
thrombocytopenia and cerebral venous sinus thrombosis after
vaccination with Ad26.COV2.S:
https://pubmed.ncbi.nlm.nih.gov/34226070/
Cerebral venous sinus thrombosis associated with
post-vaccination thrombocytopenia by COVID-19:
https://pubmed.ncbi.nlm.nih.gov/33845870/.
Cerebral venous sinus thrombosis 2 weeks after the first dose
of SARS-CoV-2 mRNA vaccine:
https://pubmed.ncbi.nlm.nih.gov/34101024/.
Vaccine-induced immune thrombotic thrombocytopenia causing a
severe form of cerebral venous thrombosis with a high mortality rate:
a case series: https://pubmed.ncbi.nlm.nih.gov/34393988/.
Adenovirus interactions with platelets and coagulation and
vaccine-associated autoimmune thrombocytopenia thrombosis syndrome:
https://pubmed.ncbi.nlm.nih.gov/34407607/.
Headache attributed to COVID-19 (SARS-CoV-2 coronavirus)
vaccination with the ChAdOx1 nCoV-19 (AZD1222) vaccine: a multicenter
observational cohort study: https://pubmed.ncbi.nlm.nih.gov/34313952/
Adverse effects reported after COVID-19 vaccination in a
tertiary care hospital, focus on cerebral venous sinus thrombosis
(CVST): https://pubmed.ncbi.nlm.nih.gov/34092166/
Cerebral venous sinus thrombosis following vaccination against
SARS-CoV-2: an analysis of cases reported to the European Medicines
Agency: https://pubmed.ncbi.nlm.nih.gov/34293217/
A rare case of a middle-age Asian male with cerebral venous
thrombosis after COVID-19 AstraZeneca vaccination:
https://pubmed.ncbi.nlm.nih.gov/34274191/
Cerebral venous sinus thrombosis negative for anti-PF4
antibody without thrombocytopenia after immunization with COVID-19
vaccine in a non-comorbid elderly Indian male treated with
conventional heparin-warfarin-based anticoagulation:
https://pubmed.ncbi.nlm.nih.gov/34186376/
Arterial events, venous thromboembolism, thrombocytopenia and
bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in
Denmark and Norway: population-based cohort study:
https://pubmed.ncbi.nlm.nih.gov/33952445/
Procoagulant microparticles: a possible link between
vaccine-induced immune thrombocytopenia (VITT) and cerebral sinus
venous thrombosis: https://pubmed.ncbi.nlm.nih.gov/34129181/
S. case reports of cerebral venous sinus thrombosis with
thrombocytopenia after vaccination with Ad26.COV2.S, March 2-April
21, 2021: https://pubmed.ncbi.nlm.nih.gov/33929487/.
Malignant cerebral infarction after vaccination with ChAdOx1
nCov-19: a catastrophic variant of vaccine-induced immune-mediated
thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34341358/
Acute ischemic stroke revealing immune thrombotic
thrombocytopenia induced by ChAdOx1 nCov-19 vaccine: impact on
recanalization strategy: https://pubmed.ncbi.nlm.nih.gov/34175640/
Vaccine-induced immune thrombotic immune thrombocytopenia
(VITT): a new clinicopathologic entity with heterogeneous clinical
presentations: https://pubmed.ncbi.nlm.nih.gov/34159588/.
Imaging and hematologic findings in thrombosis and
thrombocytopenia after vaccination with ChAdOx1 nCoV-19
(AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34402666/
Autoimmunity roots of thrombotic events after vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34508917/
Cerebral venous sinus thrombosis after vaccination: the UK
experience: https://pubmed.ncbi.nlm.nih.gov/34370974/
Massive cerebral venous thrombosis and venous basin infarction
as late complications of COVID-19: a case report:
https://pubmed.ncbi.nlm.nih.gov/34373991/
Australian and New Zealand approach to the diagnosis and
treatment of vaccine-induced immune thrombosis and immune
thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34490632/
An observational study to identify the prevalence of
thrombocytopenia and anti-PF4 / polyanion antibodies in Norwegian
health care workers after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33909350/
Acute transverse myelitis (ATM): clinical review of 43
patients with COVID-19-associated ATM and 3 serious adverse events of
post-vaccination ATM with ChAdOx1 nCoV-19 (AZD1222) vaccine:
https://pubmed.ncbi.nlm.nih.gov/33981305/.
A case of acute demyelinating polyradiculoneuropathy with
bilateral facial palsy after ChAdOx1 nCoV-19 vaccine:.
https://pubmed.ncbi.nlm.nih.gov/34272622/
Thrombocytopenia with acute ischemic stroke and hemorrhage in
a patient recently vaccinated with an adenoviral vector-based
COVID-19 vaccine:. https://pubmed.ncbi.nlm.nih.gov/33877737/
Predicted and observed incidence of thromboembolic events
among Koreans vaccinated with the ChAdOx1 nCoV-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34254476/
First dose of ChAdOx1 and BNT162b2 COVID-19 vaccines and
thrombocytopenic, thromboembolic, and hemorrhagic events in Scotland:
https://pubmed.ncbi.nlm.nih.gov/34108714/
ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: three
cases of immune thrombocytopenia after 107,720 doses of ChAdOx1
vaccination in Thailand: https://pubmed.ncbi.nlm.nih.gov/34483267/.
Pulmonary embolism, transient ischemic attack, and
thrombocytopenia after Johnson & Johnson COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34261635/
Neurosurgical considerations with respect to decompressive
craniectomy for intracerebral hemorrhage after SARS-CoV-2 vaccination
in vaccine-induced thrombotic thrombocytopenia-VITT:
https://pubmed.ncbi.nlm.nih.gov/34202817/
Large hemorrhagic stroke after vaccination against ChAdOx1
nCoV-19: a case report: https://pubmed.ncbi.nlm.nih.gov/34273119/
Polyarthralgia and myalgia syndrome after vaccination with
ChAdOx1 nCOV-19: https://pubmed.ncbi.nlm.nih.gov/34463066/
A rare case of thrombosis and thrombocytopenia of the superior
ophthalmic vein after ChAdOx1 nCoV-19 vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/34276917/
Thrombosis and severe acute respiratory syndrome Coronavirus 2
vaccines: vaccine-induced immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34237213/.
Renal vein thrombosis and pulmonary embolism secondary to
vaccine-induced thrombotic immune thrombocytopenia (VITT):
https://pubmed.ncbi.nlm.nih.gov/34268278/.
Limb ischemia and pulmonary artery thrombosis after ChAdOx1
nCoV-19 vaccine (Oxford-AstraZeneca): a case of vaccine-induced
immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/33990339/.
Association between ChAdOx1 nCoV-19 vaccination and bleeding
episodes: large population-based cohort study:
https://pubmed.ncbi.nlm.nih.gov/34479760/.
Secondary thrombocytopenia after SARS-CoV-2 vaccination: case
report of haemorrhage and hematoma after minor oral surgery:
https://pubmed.ncbi.nlm.nih.gov/34314875/.
Venous thromboembolism and mild thrombocytopenia after
vaccination with ChAdOx1 nCoV-19:
https://pubmed.ncbi.nlm.nih.gov/34384129/
Fatal exacerbation of ChadOx1-nCoV-19-induced thrombotic
thrombocytopenia syndrome after successful initial therapy with
intravenous immunoglobulins: a rationale for monitoring
immunoglobulin G levels: https://pubmed.ncbi.nlm.nih.gov/34382387/
A case of ANCA-associated vasculitis after AZD1222
(Oxford-AstraZeneca) SARS-CoV-2 vaccination: victim or causality?:
https://pubmed.ncbi.nlm.nih.gov/34416184/.
Intracerebral hemorrhage associated with vaccine-induced
thrombotic thrombocytopenia after ChAdOx1 nCOVID-19 vaccination in a
pregnant woman: https://pubmed.ncbi.nlm.nih.gov/34261297/
Massive cerebral venous thrombosis due to vaccine-induced
immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34261296/
Nephrotic syndrome after ChAdOx1 nCoV-19 vaccine against
SARScoV-2: https://pubmed.ncbi.nlm.nih.gov/34250318/.
A case of vaccine-induced immune-immune thrombotic
thrombocytopenia with massive arteriovenous thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34059191/
Cutaneous thrombosis associated with cutaneous necrosis
following Oxford-AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34189756/
Thrombocytopenia in an adolescent with sickle cell anemia
after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34331506/
Vaccine-induced thrombocytopenia with severe headache:
https://pubmed.ncbi.nlm.nih.gov/34525282/
Myocarditis associated with SARS-CoV-2 mRNA vaccination in
children aged 12 to 17 years: stratified analysis of a national
database:
https://www.medrxiv.org/content/10.1101/2021.08.30.21262866v1
COVID-19 mRNA vaccination and development of CMR-confirmed
myopericarditis:
https://www.medrxiv.org/content/10.1101/2021.09.13.21262182v1.full?s=09.
Severe autoimmune hemolytic anemia after receipt of SARS-CoV-2
mRNA vaccine: https://onlinelibrary.wiley.com/doi/10.1111/trf.16672
Intravenous injection of coronavirus disease 2019 (COVID-19)
mRNA vaccine can induce acute myopericarditis in a mouse model:
https://t.co/j0IEM8cMXI
A report of myocarditis adverse events in the U.S. Vaccine
Adverse Event Reporting System. (VAERS) in association with COVID-19
injectable biologics: https://pubmed.ncbi.nlm.nih.gov/34601006/
This study concludes that: „The vaccine was associated with
an excess risk of myocarditis (1 to 5 events per 100,000 persons).
The risk of this potentially serious adverse event and of many other
serious adverse events increased substantially after SARS-CoV-2
infection”: https://www.nejm.org/doi/full/10.1056/NEJMoa2110475
Bilateral uveitis after inoculation with COVID-19 vaccine: a
case report:
https://www.sciencedirect.com/science/article/pii/S1201971221007797
Myocarditis associated with SARS-CoV-2 mRNA vaccination in
children aged 12 to 17 years: stratified analysis of a national
database:
https://www.medrxiv.org/content/10.1101/2021.08.30.21262866v1.
Immune-mediated hepatitis with the Moderna vaccine is no
longer a coincidence but confirmed:
https://www.sciencedirect.com/science/article/pii/S0168827821020936
Extensive investigations revealed consistent pathophysiologic
alterations after vaccination with COVID-19 vaccines:
https://www.nature.com/articles/s41421-021-00329-3
Lobar hemorrhage with ventricular rupture shortly after the
first dose of an mRNA-based SARS-CoV-2 vaccine:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8553377/
Mrna COVID vaccines dramatically increase endothelial
inflammatory markers and risk of Acute Coronary Syndrome as measured
by PULS cardiac testing: a caution:
https://www.ahajournals.org/doi/10.1161/circ.144.suppl_1.10712
ChAdOx1 interacts with CAR and PF4 with implications for
thrombosis with thrombocytopenia
syndrome:https://www.science.org/doi/10.1126/sciadv.abl8213
Lethal vaccine-induced immune thrombotic immune
thrombocytopenia (VITT) following announcement 26.COV2.S: first
documented case outside the U.S.:
https://pubmed.ncbi.nlm.nih.gov/34626338/
A prothrombotic thrombocytopenic disorder resembling
heparin-induced thrombocytopenia after coronavirus-19 vaccination:
https://europepmc.org/article/PPR/PPR304469 435.
VITT (vaccine-induced immune thrombotic thrombocytopenia)
after vaccination with ChAdOx1 nCoV-19:
https://pubmed.ncbi.nlm.nih.gov/34731555/
Vaccine-induced immune thrombotic thrombocytopenia (VITT): a
new clinicopathologic entity with heterogeneous clinical
presentations: https://pubmed.ncbi.nlm.nih.gov/34159588/
Treatment of acute ischemic stroke associated with ChAdOx1
nCoV-19 vaccine-induced immune thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34461442/
Spectrum of neurological complications after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34719776/.
Cerebral venous sinus thrombosis after vaccination: the UK
experience: https://pubmed.ncbi.nlm.nih.gov/34370974/
Cerebral venous vein/venous sinus thrombosis with
thrombocytopenia syndrome after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34373413/
Portal vein thrombosis due to vaccine-induced immune
thrombotic immune thrombocytopenia (VITT) after Covid vaccination
with ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34598301/
Hematuria, a generalized petechial rash and headaches after
Oxford AstraZeneca ChAdOx1 nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34620638/
Myocardial infarction and azygos vein thrombosis after
vaccination with ChAdOx1 nCoV-19 in a hemodialysis patient:
https://pubmed.ncbi.nlm.nih.gov/34650896/
Takotsubo (stress) cardiomyopathy after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34625447/
Humoral response induced by Prime-Boost vaccination with
ChAdOx1 nCoV-19 and BNT162b2 mRNA vaccines in a patient with multiple
sclerosis treated with teriflunomide:
https://pubmed.ncbi.nlm.nih.gov/34696248/
Guillain-Barré syndrome after ChAdOx1 nCoV-19 COVID-19
vaccination: a case series: https://pubmed.ncbi.nlm.nih.gov/34548920/
Refractory vaccine-induced immune thrombotic thrombocytopenia
(VITT) treated with delayed therapeutic plasma exchange (TPE):
https://pubmed.ncbi.nlm.nih.gov/34672380/.
Rare case of COVID-19 vaccine-associated intracranial
hemorrhage with venous sinus thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34556531/.
Delayed headache after COVID-19 vaccination: a warning sign
for vaccine-induced cerebral venous thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34535076/.
Clinical features of vaccine-induced thrombocytopenia and
immune thrombosis: https://pubmed.ncbi.nlm.nih.gov/34379914/.
Predictors of mortality in thrombotic thrombocytopenia after
adenoviral COVID-19 vaccination: the FAPIC score:
https://pubmed.ncbi.nlm.nih.gov/34545400/
Ischemic stroke as a presenting feature of immune thrombotic
thrombocytopenia induced by ChAdOx1-nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34035134/
In-hospital observational study of neurological disorders in
patients recently vaccinated with COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/34688190/
Endovascular treatment for vaccine-induced cerebral venous
sinus thrombosis and thrombocytopenia after vaccination with ChAdOx1
nCoV-19: report of three cases:
https://pubmed.ncbi.nlm.nih.gov/34782400/
Cardiovascular, neurological, and pulmonary events after
vaccination with BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines:
an analysis of European data:
https://pubmed.ncbi.nlm.nih.gov/34710832/
Cerebral venous thrombosis developing after vaccination.
COVID-19: VITT, VATT, TTS and more:
https://pubmed.ncbi.nlm.nih.gov/34695859/
Cerebral venous thrombosis and myeloproliferative neoplasms: a
three-center study of 74 consecutive cases:
https://pubmed.ncbi.nlm.nih.gov/34453762/.
Possible triggers of thrombocytopenia and/or hemorrhage by
BNT162b2 vaccine, Pfizer-BioNTech:
https://pubmed.ncbi.nlm.nih.gov/34660652/.
Multiple sites of arterial thrombosis in a 35-year-old patient
after vaccination with ChAdOx1 (AstraZeneca), which required
emergency femoral and carotid surgical thrombectomy:
https://pubmed.ncbi.nlm.nih.gov/34644642/
Case series of vaccine-induced thrombotic thrombocytopenia in
a London teaching hospital: https://pubmed.ncbi.nlm.nih.gov/34694650/
Neuro-ophthalmic complications with thrombocytopenia and
thrombosis induced by ChAdOx1 nCoV-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34726934/
Thrombotic events after COVID-19 vaccination in over 50 years
of age: results of a population-based study in Italy:
https://pubmed.ncbi.nlm.nih.gov/34835237/
Intracerebral hemorrhage associated with vaccine-induced
thrombotic thrombocytopenia after ChAdOx1 nCOVID-19 vaccination in a
pregnant woman: https://pubmed.ncbi.nlm.nih.gov/34261297/
Age- and sex-specific incidence of cerebral venous sinus
thrombosis associated with Ad26.COV2.S COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34724036/.
Genital necrosis with cutaneous thrombosis following
vaccination with COVID-19 mRNA:
https://pubmed.ncbi.nlm.nih.gov/34839563/
Cerebral venous sinus thrombosis after mRNA-based COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34783932/.
COVID-19 vaccine-induced immune thrombosis with
thrombocytopenia thrombosis (VITT) and shades of gray in thrombus
formation: https://pubmed.ncbi.nlm.nih.gov/34624910/
Inflammatory myositis after vaccination with ChAdOx1:
https://pubmed.ncbi.nlm.nih.gov/34585145/
Acute ST-segment elevation myocardial infarction secondary to
vaccine-induced immune thrombosis with thrombocytopenia (VITT):
https://pubmed.ncbi.nlm.nih.gov/34580132/.
A rare case of COVID-19 vaccine-induced thrombotic
thrombocytopenia (VITT) affecting the venosplanchnic and pulmonary
arterial circulation from a UK district general hospital:
https://pubmed.ncbi.nlm.nih.gov/34535492/
COVID-19 vaccine-induced thrombotic thrombocytopenia: a case
series: https://pubmed.ncbi.nlm.nih.gov/34527501/
Thrombosis with thrombocytopenia syndrome (TTS) after
vaccination with AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19: a
risk-benefit analysis for persons <60% risk-benefit analysis for
people <60 years in Australia:
https://pubmed.ncbi.nlm.nih.gov/34272095/
Immune thrombocytopenia after immunization with Vaxzevria
ChadOx1-S vaccine (AstraZeneca), Victoria, Australia:
https://pubmed.ncbi.nlm.nih.gov/34756770/
Characteristics and outcomes of patients with cerebral venous
sinus thrombosis in thrombotic immune thrombocytopenia induced by
SARS-CoV-2 vaccine:
https://jamanetwork.com/journals/jamaneurology/fullarticle/2784622
Case study of thrombosis and thrombocytopenia syndrome after
administration of the AstraZeneca COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34781321/
Thrombosis with Thrombocytopenia Syndrome Associated with
COVID-19 Vaccines: https://pubmed.ncbi.nlm.nih.gov/34062319/
Cerebral venous sinus thrombosis following vaccination with
ChAdOx1: the first case of definite thrombosis with thrombocytopenia
syndrome in India: https://pubmed.ncbi.nlm.nih.gov/34706921/
COVID-19 vaccine-associated thrombosis with thrombocytopenia
syndrome (TTS): systematic review and post hoc analysis:
https://pubmed.ncbi.nlm.nih.gov/34698582/.
Case report of immune thrombocytopenia after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34751013/.
Acute transverse myelitis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34684047/.
Concerns for adverse effects of thrombocytopenia and
thrombosis after adenovirus-vectored COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34541935/
Major hemorrhagic stroke after ChAdOx1 nCoV-19 vaccination: a
case report: https://pubmed.ncbi.nlm.nih.gov/34273119/
Cerebral venous sinus thrombosis after COVID-19 vaccination:
neurologic and radiologic management:
https://pubmed.ncbi.nlm.nih.gov/34327553/.
Thrombocytopenia with acute ischemic stroke and hemorrhage in
a patient recently vaccinated with an adenoviral vector-based
COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/33877737/
Intracerebral hemorrhage and thrombocytopenia after
AstraZeneca COVID-19 vaccine: clinical and diagnostic challenges of
vaccine-induced thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34646685/
Minimal change disease with severe acute kidney injury after
Oxford-AstraZeneca COVID-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34242687/.
Case report: cerebral sinus vein thrombosis in two patients
with AstraZeneca SARS-CoV-2 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34609603/
Case report: Pityriasis rosea-like rash after vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34557507/
Extensive longitudinal transverse myelitis after ChAdOx1
nCOV-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34641797/.
Acute eosinophilic pneumonia associated with anti-COVID-19
vaccine AZD1222: https://pubmed.ncbi.nlm.nih.gov/34812326/.
Thrombocytopenia, including immune thrombocytopenia after
receiving COVID-19 mRNA vaccines reported to the Vaccine Adverse
Event Reporting System (VAERS):
https://pubmed.ncbi.nlm.nih.gov/34006408/
A case of ANCA-associated vasculitis after AZD1222
(Oxford-AstraZeneca) SARS-CoV-2 vaccination: victim or causality?:
https://pubmed.ncbi.nlm.nih.gov/34416184/
Vaccine-induced immune thrombosis and thrombocytopenia
syndrome after adenovirus-vectored severe acute respiratory syndrome
coronavirus 2 vaccination: a new hypothesis on mechanisms and
implications for future vaccine development:
https://pubmed.ncbi.nlm.nih.gov/34664303/.
Thrombosis in peripheral artery disease and thrombotic
thrombocytopenia following adenoviral COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34649281/.
Newly diagnosed immune thrombocytopenia in a pregnant patient
after coronavirus disease 2019 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34420249/
Cerebral venous sinus thrombosis and thrombotic events after
vector-based COVID-19 vaccines: systematic review and meta-analysis:
https://pubmed.ncbi.nlm.nih.gov/34610990/.
Sweet’s syndrome after Oxford-AstraZeneca COVID-19 vaccine
(AZD1222) in an elderly woman:
https://pubmed.ncbi.nlm.nih.gov/34590397/
Sudden sensorineural hearing loss after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34670143/.
Prevalence of serious adverse events among health care
professionals after receiving the first dose of ChAdOx1 nCoV-19
coronavirus vaccine (Covishield) in Togo, March 2021:
https://pubmed.ncbi.nlm.nih.gov/34819146/.
Acute hemichorea-hemibalismus after COVID-19 (AZD1222)
vaccination: https://pubmed.ncbi.nlm.nih.gov/34581453/
Recurrence of alopecia areata after covid-19 vaccination: a
report of three cases in Italy:
https://pubmed.ncbi.nlm.nih.gov/34741583/
Shingles-like skin lesion after vaccination with AstraZeneca
for COVID-19: a case report:
https://pubmed.ncbi.nlm.nih.gov/34631069/
Thrombosis after COVID-19 vaccination: possible link to ACE
pathways: https://pubmed.ncbi.nlm.nih.gov/34479129/
Thrombocytopenia in an adolescent with sickle cell anemia
after COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34331506/
Leukocytoclastic vasculitis as a cutaneous manifestation of
ChAdOx1 corona virus vaccine nCoV-19 (recombinant):
https://pubmed.ncbi.nlm.nih.gov/34546608/
Abdominal pain and bilateral adrenal hemorrhage from immune
thrombotic thrombocytopenia induced by COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34546343/
Longitudinally extensive cervical myelitis after vaccination
with inactivated virus based COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34849183/
Induction of cutaneous leukocytoclastic vasculitis after
ChAdOx1 nCoV-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34853744/.
A case of toxic epidermal necrolysis after vaccination with
ChAdOx1 nCoV-19 (AZD1222): https://pubmed.ncbi.nlm.nih.gov/34751429/.
Ocular adverse events following COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34559576/
Depression after ChAdOx1-S / nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34608345/.
Venous thromboembolism and mild thrombocytopenia after ChAdOx1
nCoV-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34384129/.
Recurrent ANCA-associated vasculitis after Oxford AstraZeneca
ChAdOx1-S COVID-19 vaccination: a case series of two patients:
https://pubmed.ncbi.nlm.nih.gov/34755433/
Major artery thrombosis and vaccination against ChAdOx1
nCov-19: https://pubmed.ncbi.nlm.nih.gov/34839830/
Rare case of contralateral supraclavicular lymphadenopathy
after vaccination with COVID-19: computed tomography and ultrasound
findings: https://pubmed.ncbi.nlm.nih.gov/34667486/
Cutaneous lymphocytic vasculitis after administration of the
second dose of AZD1222 (Oxford-AstraZeneca) Severe acute respiratory
syndrome Coronavirus 2 vaccine: chance or causality:
https://pubmed.ncbi.nlm.nih.gov/34726187/.
Pancreas allograft rejection after ChAdOx1 nCoV-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34781027/
Understanding the risk of thrombosis with thrombocytopenia
syndrome following Ad26.COV2.S vaccination:
https://pubmed.ncbi.nlm.nih.gov/34595694/
Cutaneous adverse reactions of 35,229 doses of COVID-19
Sinovac and AstraZeneca vaccine COVID-19: a prospective cohort study
in health care workers: https://pubmed.ncbi.nlm.nih.gov/34661934/
Comments on thrombosis after vaccination: spike protein leader
sequence could be responsible for thrombosis and antibody-mediated
thrombocytopenia: https://pubmed.ncbi.nlm.nih.gov/34788138
Eosinophilic dermatosis after AstraZeneca COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34753210/.
Severe immune thrombocytopenia following COVID-19 vaccination:
report of four cases and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34653943/.
Relapse of immune thrombocytopenia after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34591991/
Thrombosis in pre- and post-vaccination phase of COVID-19;
https://pubmed.ncbi.nlm.nih.gov/34650382/
A look at the role of postmortem immunohistochemistry in
understanding the inflammatory pathophysiology of COVID-19 disease
and vaccine-related thrombotic adverse events: a narrative review:
https://pubmed.ncbi.nlm.nih.gov/34769454/
COVID-19 vaccine in patients with hypercoagulability
disorders: a clinical perspective:
https://pubmed.ncbi.nlm.nih.gov/34786893/
Vaccine-associated thrombocytopenia and thrombosis: venous
endotheliopathy leading to combined venous micro-macrothrombosis:
https://pubmed.ncbi.nlm.nih.gov/34833382/
Thrombosis and thrombocytopenia syndrome causing isolated
symptomatic carotid occlusion after COVID-19 Ad26.COV2.S vaccine
(Janssen): https://pubmed.ncbi.nlm.nih.gov/34670287/
An unusual presentation of acute deep vein thrombosis after
Modern COVID-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34790811/
Immediate high-dose intravenous immunoglobulins followed by
direct treatment with thrombin inhibitors is crucial for survival in
vaccine-induced immune thrombotic thrombocytopenia
Sars-Covid-19-vector adenoviral VITT with venous thrombosis of the
cerebral sinus and portal vein:
https://pubmed.ncbi.nlm.nih.gov/34023956/.
Thrombosis formation after COVID-19 vaccination immunologic
aspects: review article: https://pubmed.ncbi.nlm.nih.gov/34629931/
Imaging and hematologic findings in thrombosis and
thrombocytopenia after vaccination with ChAdOx1 nCoV-19
(AstraZeneca): https://pubmed.ncbi.nlm.nih.gov/34402666/
Spectrum of neuroimaging findings in post-CoVID-19
vaccination: a case series and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34842783/
Cerebral venous sinus thrombosis, pulmonary embolism, and
thrombocytopenia after COVID-19 vaccination in a Taiwanese man: a
case report and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34630307/
Fatal cerebral venous sinus thrombosis after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33983464/
Autoimmune roots of thrombotic events after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34508917/.
New portal vein thrombosis in cirrhosis: is thrombophilia
exacerbated by vaccine or COVID-19:
https://www.jcehepatology.com/article/S0973-6883(21)00545-4/fulltext.
Images of immune thrombotic thrombocytopenia induced by Oxford
/ AstraZeneca® COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33962903/.
Cerebral venous sinus thrombosis after vaccination with
COVID-19 mRNA of BNT162b2: https://pubmed.ncbi.nlm.nih.gov/34796065/.
Increased risk of urticaria/angioedema after BNT162b2 mRNA
COVID-19 vaccination in health care workers taking ACE inhibitors:
https://pubmed.ncbi.nlm.nih.gov/34579248/
A case of unusual mild clinical presentation of COVID-19
vaccine-induced immune thrombotic thrombocytopenia with splanchnic
vein thrombosis: https://pubmed.ncbi.nlm.nih.gov/34843991/
Cerebral venous sinus thrombosis following vaccination with
Pfizer-BioNTech COVID-19 (BNT162b2):
https://pubmed.ncbi.nlm.nih.gov/34595867/
A case of idiopathic thrombocytopenic purpura after a booster
dose of COVID-19 BNT162b2 vaccine (Pfizer-Biontech):
https://pubmed.ncbi.nlm.nih.gov/34820240/
Vaccine-induced immune thrombotic immune thrombocytopenia
(VITT): targeting pathologic mechanisms with Bruton’s tyrosine
kinase inhibitors: https://pubmed.ncbi.nlm.nih.gov/33851389/
Thrombotic thrombocytopenic purpura after vaccination with
Ad26.COV2-S: https://pubmed.ncbi.nlm.nih.gov/33980419/
Thromboembolic events in younger females exposed to
Pfizer-BioNTech or Moderna COVID-19 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34264151/
Potential risk of thrombotic events after COVID-19 vaccination
with Oxford-AstraZeneca in women receiving estrogen:
https://pubmed.ncbi.nlm.nih.gov/34734086/
Thrombosis after adenovirus-vectored COVID-19 vaccination: a
concern for underlying disease:
https://pubmed.ncbi.nlm.nih.gov/34755555/
Adenovirus interactions with platelets and coagulation and
vaccine-induced immune thrombotic thrombocytopenia syndrome:
https://pubmed.ncbi.nlm.nih.gov/34407607/
Thrombotic thrombocytopenic purpura: a new threat after COVID
bnt162b2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34264514/.
Unusual site of deep vein thrombosis after vaccination against
coronavirus mRNA-2019 coronavirus disease (COVID-19):
https://pubmed.ncbi.nlm.nih.gov/34840204/
Neurological side effects of SARS-CoV-2 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34750810/
Coagulopathies after SARS-CoV-2 vaccination may derive from a
combined effect of SARS-CoV-2 spike protein and adenovirus
vector-activated signaling pathways:
https://pubmed.ncbi.nlm.nih.gov/34639132/
Isolated pulmonary embolism after COVID vaccination: 2 case
reports and a review of acute pulmonary embolism complications and
follow-up: https://pubmed.ncbi.nlm.nih.gov/34804412/
Central retinal vein occlusion after vaccination with
SARS-CoV-2 mRNA: case report:
https://pubmed.ncbi.nlm.nih.gov/34571653/.
Complicated case report of long-term vaccine-induced
thrombotic immune thrombocytopenia A:
https://pubmed.ncbi.nlm.nih.gov/34835275/.
Deep venous thrombosis after vaccination with Ad26.COV2.S in
adult males: https://pubmed.ncbi.nlm.nih.gov/34659839/.
Neurological autoimmune diseases after SARS-CoV-2 vaccination:
a case series: https://pubmed.ncbi.nlm.nih.gov/34668274/.
Severe autoimmune hemolytic autoimmune anemia after receiving
SARS-CoV-2 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34549821/
Occurrence of COVID-19 variants among recipients of ChAdOx1
nCoV-19 vaccine (recombinant):
https://pubmed.ncbi.nlm.nih.gov/34528522/
Prevalence of thrombocytopenia, anti-platelet factor 4
antibodies, and elevated D-dimer in Thais after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34568726/
Epidemiology of acute myocarditis/pericarditis in Hong Kong
adolescents after co-vaccination:
https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciab989/644
5179.
Myocarditis after 2019 coronavirus disease mRNA vaccine: a
case series and determination of incidence rate:
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab926/6420408
Myocarditis and pericarditis after COVID-19 vaccination:
inequalities in age and vaccine types:
https://www.mdpi.com/2075-4426/11/11/1106
Epidemiology and clinical features of myocarditis/pericarditis
before the introduction of COVID-19 mRNA vaccine in Korean children:
a multicenter study: https://pubmed.ncbi.nlm.nih.gov/34402230/
Shedding light on post-vaccination myocarditis and
pericarditis in COVID-19 and non-COVID-19 vaccine recipients:
https://pubmed.ncbi.nlm.nih.gov/34696294/
Myocarditis Following mRNA COVID-19 Vaccine:
https://journals.lww.com/pec-online/Abstract/2021/11000/Myocarditis_Following_
mRNA_COVID_19_Vaccine.9.aspx.
Myocarditis following BNT162b2 mRNA Covid-19 mRNA vaccine in
Israel: https://pubmed.ncbi.nlm.nih.gov/34614328/.
Myocarditis, pericarditis, and cardiomyopathy following
COVID-19 vaccination:
https://www.heartlungcirc.org/article/S1443-9506(21)01156-2/fulltext
Myocarditis and other cardiovascular complications of COVID-19
mRNA-based COVID-19 vaccines:
https://pubmed.ncbi.nlm.nih.gov/34277198/
Possible Association Between COVID-19 Vaccine and Myocarditis:
Clinical and CMR Findings: https://pubmed.ncbi.nlm.nih.gov/34246586/
Hypersensitivity Myocarditis and COVID-19 Vaccines:
https://pubmed.ncbi.nlm.nih.gov/34856634/.
Severe myocarditis associated with COVID-19 vaccine: zebra or
unicorn?:
https://www.internationaljournalofcardiology.com/article/S0167-5273(21)01477-7/fulltext.
Acute myocardial infarction and myocarditis after COVID-19
vaccination:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8522388/
Myocarditis after Covid-19 vaccination in a large healthcare
organization: https://www.nejm.org/doi/10.1056/NEJMoa2110737
Association of myocarditis with COVID-19 messenger RNA
BNT162b2 vaccine in a case series of children:
https://jamanetwork.com/journals/jamacardiology/fullarticle/2783052
Clinical suspicion of myocarditis temporally related to
COVID-19 vaccination in adolescents and young adults:
https://www.ahajournals.org/doi/abs/10.1161/CIRCULATIONAHA.121.056583?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
STEMI mimicry: focal myocarditis in an adolescent patient
after COVID-19 mRNA vaccination:.
https://pubmed.ncbi.nlm.nih.gov/34756746/
Myocarditis and pericarditis in association with COVID-19 mRNA
vaccination: cases from a regional pharmacovigilance center:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8587334/
Myocarditis after COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/34546329/.
Patients with acute myocarditis after COVID-19 mRNA
vaccination:.
https://jamanetwork.com/journals/jamacardiology/fullarticle/2781602.
Myocarditis after COVID-19 vaccination: a case series:
https://www.sciencedirect.com/science/article/pii/S0264410X21011725?via%3Dihub.
Myocarditis associated with COVID-19 vaccination in
adolescents:
https://publications.aap.org/pediatrics/article/148/5/e2021053427/181357
Myocarditis findings on cardiac magnetic resonance imaging
after vaccination with COVID-19 mRNA in adolescents:.
https://pubmed.ncbi.nlm.nih.gov/34704459/
Myocarditis after COVID-19 vaccination: magnetic resonance
imaging study:
https://academic.oup.com/ehjcimaging/advance-article/doi/10.1093/ehjci/jeab230/6
421640.
Acute myocarditis after administration of the second dose of
BNT162b2 COVID-19 vaccine:
https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8599115/
Myocarditis after COVID-19 vaccination:
https://www.sciencedirect.com/science/article/pii/S2352906721001603
Case report: probable myocarditis after Covid-19 mRNA vaccine
in a patient with arrhythmogenic left ventricular cardiomyopathy:
https://pubmed.ncbi.nlm.nih.gov/34712717/.
Acute myocarditis after administration of BNT162b2 vaccine
against COVID-19:
https://www.revespcardiol.org/en-linkresolver-acute-myocarditis-after-administration-bnt162b2-S188558572100133X.
Myocarditis associated with COVID-19 mRNA vaccination:
https://pubs.rsna.org/doi/10.1148/radiol.2021211430
Acute myocarditis after COVID-19 vaccination: a case report:
https://www.sciencedirect.com/science/article/pii/S0248866321007098
Acute myopericarditis after COVID-19 vaccination in
adolescents:. https://pubmed.ncbi.nlm.nih.gov/34589238/.
Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19
vaccination:
https://academic.oup.com/jpids/article/10/10/962/6329543.
Acute myocarditis associated with anti-COVID-19 vaccination:
https://ecevr.org/DOIx.php?id=10.7774/cevr.2021.10.2.196.
Myocarditis associated with COVID-19 vaccination:
echocardiographic, cardiac CT, and MRI findings:.
https://pubmed.ncbi.nlm.nih.gov/34428917/.
Acute symptomatic myocarditis in 7 adolescents after
Pfizer-BioNTech COVID-19 vaccination:.
https://pubmed.ncbi.nlm.nih.gov/34088762/.
Myocarditis and pericarditis in adolescents after first and
second doses of COVID-19 mRNA vaccines:.
https://academic.oup.com/ehjqcco/advance-article/doi/10.1093/ehjqcco/qcab090/64
42104.
COVID 19 vaccine for adolescents. Concern for myocarditis and
pericarditis: https://www.mdpi.com/2036-7503/13/3/61.
Cardiac imaging of acute myocarditis after vaccination with
COVID-19 mRNA: https://pubmed.ncbi.nlm.nih.gov/34402228/
Myocarditis temporally associated with COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34133885/
Acute myocardial injury after COVID-19 vaccination: a case
report and review of current evidence from the vaccine adverse event
reporting system database: https://pubmed.ncbi.nlm.nih.gov/34219532/
Acute myocarditis associated with COVID-19 vaccination: report
of a case: https://www.ncbi.nlm.nih.gov/labs/pmc/articles/PMC8639400/
Myocarditis following vaccination with COVID-19 messenger RNA:
a Japanese case series: https://pubmed.ncbi.nlm.nih.gov/34840235/.
Myocarditis in the setting of a recent COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34712497/.
Acute myocarditis after a second dose of COVID-19 mRNA
vaccine: report of two cases:
https://www.clinicalimaging.org/article/S0899-7071(21)00265-5/fulltext.
Prevalence of thrombocytopenia, antiplatelet factor 4
antibodies, and elevated D-dimer in Thais after vaccination with
ChAdOx1 nCoV-19: https://pubmed.ncbi.nlm.nih.gov/34568726/
Epidemiology of acute myocarditis/pericarditis in Hong Kong
adolescents after co-vaccination:
https://academic.oup.com/cid/advance-article-abstract/doi/10.1093/cid/ciab989/6445179
Myocarditis after 2019 coronavirus disease mRNA vaccine: a
case series and incidence rate determination:
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab926/6420408.
Myocarditis and pericarditis after COVID-19 vaccination:
inequalities in age and vaccine types:
https://www.mdpi.com/2075-4426/11/11/1106
Epidemiology and clinical features of myocarditis/pericarditis
before the introduction of COVID-19 mRNA vaccine in Korean children:
a multicenter study: https://pubmed.ncbi.nlm.nih.gov/34402230/
Shedding light on post-vaccination myocarditis and
pericarditis in COVID-19 and non-COVID-19 vaccine recipients:
https://pubmed.ncbi.nlm.nih.gov/34696294/
Diffuse prothrombotic syndrome after administration of ChAdOx1
nCoV-19 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34615534/
Three cases of acute venous thromboembolism in women after
coronavirus 2019 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34352418/
Clinical and biological features of cerebral venous sinus
thrombosis after vaccination with ChAdOx1 nCov-19;
https://jnnp.bmj.com/content/early/2021/09/29/jnnp-2021-327340.
COV2-S vaccination may reveal hereditary thrombophilia:
massive cerebral venous sinus thrombosis in a young man with normal
platelet count: https://pubmed.ncbi.nlm.nih.gov/34632750/
Post-mortem findings in vaccine-induced thrombotic
thrombocytopenia:
https://haematologica.org/article/view/haematol.2021.279075
COVID-19 vaccine-induced thrombosis:
https://pubmed.ncbi.nlm.nih.gov/34802488/.
Inflammation and platelet activation after COVID-19 vaccines:
possible mechanisms behind vaccine-induced immune thrombocytopenia
and thrombosis: https://pubmed.ncbi.nlm.nih.gov/34887867/.
Anaphylactoid reaction and coronary thrombosis related to
COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34863404/.
Vaccine-induced cerebral venous thrombosis and
thrombocytopenia. Oxford-AstraZeneca COVID-19: a missed opportunity
for rapid return on experience:
https://www.sciencedirect.com/science/article/pii/S235255682100093X
Occurrence of splenic infarction due to arterial thrombosis
after vaccination with COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34876440/
Deep venous thrombosis more than two weeks after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33928773/
Case report: Take a second look: Cerebral venous thrombosis
related to Covid-19 vaccination and thrombotic thrombocytopenia
syndrome: https://pubmed.ncbi.nlm.nih.gov/34880826/
Information on ChAdOx1 nCoV-19 vaccine-induced immune-mediated
thrombotic thrombocytopenia:
https://pubmed.ncbi.nlm.nih.gov/34587242/
Change in blood viscosity after COVID-19 vaccination:
estimation for persons with underlying metabolic syndrome:
https://pubmed.ncbi.nlm.nih.gov/34868465/
Management of a patient with a rare congenital limb
malformation syndrome after SARS-CoV-2 vaccine-induced thrombosis and
thrombocytopenia (VITT): https://pubmed.ncbi.nlm.nih.gov/34097311/
Bilateral thalamic stroke: a case of COVID-19 (VITT)
vaccine-induced immune thrombotic thrombocytopenia or a coincidence
due to underlying risk factors:
https://pubmed.ncbi.nlm.nih.gov/34820232/.
Thrombocytopenia and splanchnic thrombosis after vaccination
with Ad26.COV2.S successfully treated with transjugular intrahepatic
intrahepatic portosystemic shunt and thrombectomy:
https://onlinelibrary.wiley.com/doi/10.1002/ajh.26258
Incidence of acute ischemic stroke after coronavirus
vaccination in Indonesia: case series:
https://pubmed.ncbi.nlm.nih.gov/34579636/
Successful treatment of vaccine-induced immune immune
thrombotic thrombocytopenia in a 26-year-old female patient:
https://pubmed.ncbi.nlm.nih.gov/34614491/
Case report: vaccine-induced immune immune thrombotic
thrombocytopenia in a patient with pancreatic cancer after
vaccination with messenger RNA-1273:
https://pubmed.ncbi.nlm.nih.gov/34790684/
Idiopathic idiopathic external jugular vein thrombophlebitis
after coronavirus disease vaccination (COVID-19):
https://pubmed.ncbi.nlm.nih.gov/33624509/.
Squamous cell carcinoma of the lung with hemoptysis following
vaccination with tozinameran (BNT162b2, Pfizer-BioNTech):
https://pubmed.ncbi.nlm.nih.gov/34612003/
Vaccine-induced thrombotic thrombocytopenia after Ad26.COV2.S
vaccination in a man presenting as acute venous thromboembolism:
https://pubmed.ncbi.nlm.nih.gov/34096082/
Myocarditis associated with COVID-19 vaccination in three
adolescent boys: https://pubmed.ncbi.nlm.nih.gov/34851078/.
Cardiovascular magnetic resonance findings in young adult
patients with acute myocarditis after COVID-19 mRNA vaccination: a
case series: https://pubmed.ncbi.nlm.nih.gov/34496880/
Perimyocarditis after vaccination with COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34866957/
Epidemiology of acute myocarditis/pericarditis in Hong Kong
adolescents after co-vaccination:
https://pubmed.ncbi.nlm.nih.gov/34849657/.
Myocarditis-induced sudden death after BNT162b2 COVID-19 mRNA
vaccination in Korea: case report focusing on histopathological
findings: https://pubmed.ncbi.nlm.nih.gov/34664804/
Acute myocarditis after vaccination with COVID-19 mRNA in
adults aged 18 years or older:
https://pubmed.ncbi.nlm.nih.gov/34605853/
Recurrence of acute myocarditis temporally associated with
receipt of the 2019 coronavirus mRNA disease vaccine (COVID-19) in an
adolescent male: https://pubmed.ncbi.nlm.nih.gov/34166671/
Young male with myocarditis after mRNA-1273 coronavirus
disease-2019 (COVID-19) mRNA vaccination:
https://pubmed.ncbi.nlm.nih.gov/34744118/
Acute myocarditis after SARS-CoV-2 vaccination in a
24-year-old male: https://pubmed.ncbi.nlm.nih.gov/34334935/.
Ga-DOTATOC digital PET images of inflammatory cell infiltrates
in myocarditis after vaccination with COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34746968/
Occurrence of acute infarct-like myocarditis after vaccination
with COVID-19: just an accidental coincidence or rather a
vaccination-associated autoimmune myocarditis?”:
https://pubmed.ncbi.nlm.nih.gov/34333695/.
Self-limited myocarditis presenting with chest pain and
ST-segment elevation in adolescents after vaccination with BNT162b2
mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34180390/
Myocarditis Following Immunization with COVID-19 mRNA Vaccines
in Members of the U.S. Military:
https://pubmed.ncbi.nlm.nih.gov/34185045/
Myocarditis after BNT162b2 vaccination in a healthy male:
https://pubmed.ncbi.nlm.nih.gov/34229940/
Myopericarditis in a previously healthy adolescent male after
COVID-19 vaccination: Case report:
https://pubmed.ncbi.nlm.nih.gov/34133825/
Acute myocarditis after SARS-CoV-2 mRNA-1273 mRNA vaccination:
https://pubmed.ncbi.nlm.nih.gov/34308326/.
Chest pain with abnormal electrocardiogram redevelopment after
injection of COVID-19 vaccine manufactured by Moderna:
https://pubmed.ncbi.nlm.nih.gov/34866106/
Biopsy-proven lymphocytic myocarditis after first vaccination
with COVID-19 mRNA in a 40-year-old man: case report:
https://pubmed.ncbi.nlm.nih.gov/34487236/
Multimodality imaging and histopathology in a young man
presenting with fulminant lymphocytic myocarditis and cardiogenic
shock after vaccination with mRNA-1273:
https://pubmed.ncbi.nlm.nih.gov/34848416/
Report of a case of myopericarditis after vaccination with
BNT162b2 COVID-19 mRNA in a young Korean male:
https://pubmed.ncbi.nlm.nih.gov/34636504/
Acute myocarditis after Comirnaty vaccination in a healthy
male with previous SARS-CoV-2 infection:
https://pubmed.ncbi.nlm.nih.gov/34367386/
Acute myocarditis in a young adult two days after vaccination
with Pfizer: https://pubmed.ncbi.nlm.nih.gov/34709227/
Case report: acute fulminant myocarditis and cardiogenic shock
after messenger RNA coronavirus vaccination in 2019 requiring
extracorporeal cardiopulmonary resuscitation:
https://pubmed.ncbi.nlm.nih.gov/34778411/
Acute myocarditis after 2019 coronavirus disease vaccination:
https://pubmed.ncbi.nlm.nih.gov/34734821/
A series of patients with myocarditis after vaccination
against SARS-CoV-2 with mRNA-1279 and BNT162b2:
https://pubmed.ncbi.nlm.nih.gov/34246585/
Myopericarditis after Pfizer messenger ribonucleic acid
coronavirus coronavirus disease vaccine in adolescents:
https://pubmed.ncbi.nlm.nih.gov/34228985/
Post-vaccination multisystem inflammatory syndrome in adults
without evidence of prior SARS-CoV-2 infection:
https://pubmed.ncbi.nlm.nih.gov/34852213/
Acute myocarditis defined after vaccination with 2019 mRNA of
coronavirus disease: https://pubmed.ncbi.nlm.nih.gov/34866122/
Biventricular systolic dysfunction in acute myocarditis after
SARS-CoV-2 mRNA-1273 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34601566/
Myocarditis following COVID-19 vaccination: MRI study:
https://pubmed.ncbi.nlm.nih.gov/34739045/.
Acute myocarditis after COVID-19 vaccination: case report:
https://docs.google.com/document/d/1Hc4bh_qNbZ7UVm5BLxkRdMPnnI9zcCsl/e
Association of myocarditis with COVID-19 messenger RNA
BNT162b2 vaccine COVID-19 in a case series of children:
https://pubmed.ncbi.nlm.nih.gov/34374740/
Clinical suspicion of myocarditis temporally related to
COVID-19 vaccination in adolescents and young adults:
https://pubmed.ncbi.nlm.nih.gov/34865500/
Myocarditis following vaccination with Covid-19 in a large
healthcare organization: https://pubmed.ncbi.nlm.nih.gov/34614329/
AstraZeneca COVID-19 vaccine and Guillain-Barré syndrome in
Tasmania: a causal link: https://pubmed.ncbi.nlm.nih.gov/34560365/
COVID-19, Guillain-Barré and vaccineA dangerous mix:
https://pubmed.ncbi.nlm.nih.gov/34108736/.
Guillain-Barré syndrome after the first dose of
Pfizer-BioNTech COVID-19 vaccine: case report and review of reported
cases: https://pubmed.ncbi.nlm.nih.gov/34796417/.
Guillain-Barre syndrome after BNT162b2 COVID-19 vaccine:
https://link.springer.com/article/10.1007%2Fs10072-021-05523-5.
COVID-19 adenovirus vaccines and Guillain-Barré syndrome with
facial palsy: https://onlinelibrary.wiley.com/doi/10.1002/ana.26258.
Association of receipt association of Ad26.COV2.S COVID-19
vaccine with presumed Guillain-Barre syndrome, February-July 2021:
https://jamanetwork.com/journals/jama/fullarticle/2785009
A case of Guillain-Barré syndrome after Pfizer COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34567447/
Guillain-Barré syndrome associated with COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34648420/.
Rate of recurrent Guillain-Barré syndrome after COVID-19
BNT162b2 mRNA vaccine:
https://jamanetwork.com/journals/jamaneurology/fullarticle/2783708
Guillain-Barre syndrome after COVID-19 vaccination in an
adolescent:
https://www.pedneur.com/article/S0887-8994(21)00221-6/fulltext.
Guillain-Barre syndrome after ChAdOx1-S / nCoV-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34114256/.
Guillain-Barre syndrome after COVID-19 mRNA-1273 vaccine: case
report: https://pubmed.ncbi.nlm.nih.gov/34767184/.
Guillain-Barre syndrome following SARS-CoV-2 vaccination in 19
patients: https://pubmed.ncbi.nlm.nih.gov/34644738/.
Guillain-Barre syndrome presenting with facial diplegia
following vaccination with COVID-19 in two patients:
https://pubmed.ncbi.nlm.nih.gov/34649856/
A rare case of Guillain-Barré syndrome after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34671572/
Neurological complications of COVID-19: Guillain-Barre
syndrome after Pfizer COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33758714/
COVID-19 vaccine causing Guillain-Barre syndrome, an uncommon
potential side effect: https://pubmed.ncbi.nlm.nih.gov/34484780/
Guillain-Barre syndrome after the first dose of COVID-19
vaccination: case report; https://pubmed.ncbi.nlm.nih.gov/34779385/.
Miller Fisher syndrome after Pfizer COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34817727/.
Miller Fisher syndrome after 2019 BNT162b2 mRNA coronavirus
vaccination: https://pubmed.ncbi.nlm.nih.gov/34789193/.
Bilateral facial weakness with a variant of paresthesia of
Guillain-Barre syndrome after Vaxzevria COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34261746/
Guillain-Barre syndrome after the first injection of ChAdOx1
nCoV-19 vaccine: first report:
https://pubmed.ncbi.nlm.nih.gov/34217513/.
A case of sensory ataxic Guillain-Barre syndrome with
immunoglobulin G anti-GM1 antibodies after first dose of COVID-19
BNT162b2 mRNA vaccine (Pfizer):
https://pubmed.ncbi.nlm.nih.gov/34871447/
Reporting of acute inflammatory neuropathies with COVID-19
vaccines: subgroup disproportionality analysis in VigiBase:
https://pubmed.ncbi.nlm.nih.gov/34579259/
A variant of Guillain-Barré syndrome after SARS-CoV-2
vaccination: AMSAN: https://pubmed.ncbi.nlm.nih.gov/34370408/.
A rare variant of Guillain-Barré syndrome after vaccination
with Ad26.COV2.S: https://pubmed.ncbi.nlm.nih.gov/34703690/.
Guillain-Barré syndrome after SARS-CoV-2 vaccination in a
patient with previous vaccine-associated Guillain-Barré syndrome:
https://pubmed.ncbi.nlm.nih.gov/34810163/
Guillain-Barré syndrome in an Australian state using mRNA and
adenovirus-vector SARS-CoV-2 vaccines:
https://onlinelibrary.wiley.com/doi/10.1002/ana.26218.
Acute transverse myelitis after SARS-CoV-2 vaccination: case
report and review of the literature:
https://pubmed.ncbi.nlm.nih.gov/34482455/.
Variant Guillain-Barré syndrome occurring after SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34114269/.
Guillian-Barre syndrome with axonal variant temporally
associated with Modern SARS-CoV-2 mRNA-based vaccine:
https://pubmed.ncbi.nlm.nih.gov/34722067/
Guillain-Barre syndrome after the first dose of SARS-CoV-2
vaccine: a temporary occurrence, not a causal association:
https://pubmed.ncbi.nlm.nih.gov/33968610/
SARS-CoV-2 vaccines can be complicated not only by
Guillain-Barré syndrome but also by distal small fiber neuropathy:
https://pubmed.ncbi.nlm.nih.gov/34525410/
Clinical variant of Guillain-Barré syndrome with prominent
facial diplegia after AstraZeneca 2019 coronavirus disease vaccine:
https://pubmed.ncbi.nlm.nih.gov/34808658/
Adverse event reporting and risk of Bell’s palsy after
COVID-19 vaccination:
https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(21)00646-0/fulltext.
Bilateral facial nerve palsy and COVID-19 vaccination:
causality or coincidence: https://pubmed.ncbi.nlm.nih.gov/34522557/
Left Bell’s palsy after the first dose of mRNA-1273
SARS-CoV-2 vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34763263/.
Bell’s palsy after inactivated vaccination with COVID-19 in
a patient with a history of recurrent Bell’s palsy: case report:
https://pubmed.ncbi.nlm.nih.gov/34621891/
Neurological complications after the first dose of COVID-19
vaccines and SARS-CoV-2 infection:
https://pubmed.ncbi.nlm.nih.gov/34697502/
Type I interferons as a potential mechanism linking COVID-19
mRNA vaccines with Bell’s palsy:
https://pubmed.ncbi.nlm.nih.gov/33858693/
Acute transverse myelitis following inactivated COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34370410/
Acute transverse myelitis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34579245/.
A case of longitudinally extensive transverse myelitis
following Covid-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34182207/
Post COVID-19 transverse myelitis; a case report with review
of the literature: https://pubmed.ncbi.nlm.nih.gov/34457267/.
Beware of neuromyelitis optica spectrum disorder after
vaccination with inactivated virus for COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34189662/
Neuromyelitis optica in a healthy woman after vaccination
against severe acute respiratory syndrome coronavirus 2 mRNA-1273:
https://pubmed.ncbi.nlm.nih.gov/34660149/
Acute bilateral bilateral optic neuritis/chiasm with
longitudinal extensive transverse myelitis in long-standing stable
multiple sclerosis after vector-based vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/34131771/
A case series of acute pericarditis after vaccination with
COVID-19 in the context of recent reports from Europe and the United
States: https://pubmed.ncbi.nlm.nih.gov/34635376/
Acute pericarditis and cardiac tamponade after vaccination
with Covid-19: https://pubmed.ncbi.nlm.nih.gov/34749492/
Myocarditis and pericarditis in adolescents after the first
and second doses of COVID-19 mRNA vaccines:
https://pubmed.ncbi.nlm.nih.gov/34849667/
Perimyocarditis in adolescents after Pfizer-BioNTech COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34319393/
Acute myopericarditis after COVID-19 vaccine in adolescents:
https://pubmed.ncbi.nlm.nih.gov/34589238/
Pericarditis after administration of the BNT162b2 mRNA vaccine
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34149145/
Case report: symptomatic pericarditis post COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34693198/.
An outbreak of Still’s disease after COVID-19 vaccination in
a 34-year-old patient: https://pubmed.ncbi.nlm.nih.gov/34797392/
Hemophagocytic lymphohistiocytosis following COVID-19
vaccination (ChAdOx1 nCoV-19):
https://pubmed.ncbi.nlm.nih.gov/34862234/
Myocarditis after SARS-CoV-2 mRNA vaccination, a case series:
https://pubmed.ncbi.nlm.nih.gov/34396358/.
Miller-Fisher syndrome and Guillain-Barré syndrome overlap
syndrome in a patient after Oxford-AstraZeneca SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34848426/.
Immune-mediated disease outbreaks or new-onset disease in 27
subjects after mRNA/DNA vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/33946748/
Post-mortem investigation of deaths after vaccination with
COVID-19 vaccines: https://pubmed.ncbi.nlm.nih.gov/34591186/
Acute kidney injury with macroscopic hematuria and IgA
nephropathy after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34352309/
Relapse of immune thrombocytopenia after covid-19 vaccination
in young male patient: https://pubmed.ncbi.nlm.nih.gov/34804803/.
Immune thrombocytopenic purpura associated with COVID-19 mRNA
vaccine Pfizer-BioNTech BNT16B2b2:
https://pubmed.ncbi.nlm.nih.gov/34077572/
Retinal hemorrhage after SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34884407/.
Case report: anti-neutrophil cytoplasmic antibody-associated
vasculitis with acute renal failure and pulmonary hemorrhage can
occur after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34859017/
Intracerebral hemorrhage due to vasculitis following COVID-19
vaccination: case report: https://pubmed.ncbi.nlm.nih.gov/34783899/
Peduncular, symptomatic cavernous bleeding after immune
thrombocytopenia-induced SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34549178/.
Brain death in a vaccinated patient with COVID-19 infection:
https://pubmed.ncbi.nlm.nih.gov/34656887/
Generalized purpura annularis telangiectodes after SARS-CoV-2
mRNA vaccination: https://pubmed.ncbi.nlm.nih.gov/34236717/.
Lobar hemorrhage with ventricular rupture shortly after the
first dose of a SARS-CoV-2 mRNA-based SARS-CoV-2 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34729467/.
A case of outbreak of macroscopic hematuria and IgA
nephropathy after SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33932458/
Acral hemorrhage after administration of the second dose of
SARS-CoV-2 vaccine. A post-vaccination reaction:
https://pubmed.ncbi.nlm.nih.gov/34092400/742.
Severe immune thrombocytopenic purpura after SARS-CoV-2
vaccine: https://pubmed.ncbi.nlm.nih.gov/34754937/
Gross hematuria after severe acute respiratory syndrome
coronavirus 2 vaccination in 2 patients with IgA nephropathy:
https://pubmed.ncbi.nlm.nih.gov/33771584/
Autoimmune encephalitis after ChAdOx1-S SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34846583/
COVID-19 vaccine and death: causality algorithm according to
the WHO eligibility diagnosis:
https://pubmed.ncbi.nlm.nih.gov/34073536/
Bell’s palsy after vaccination with mRNA (BNT162b2) and
inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and a
nested case-control study: https://pubmed.ncbi.nlm.nih.gov/34411532/
Epidemiology of myocarditis and pericarditis following mRNA
vaccines in Ontario, Canada: by vaccine product, schedule, and
interval:
https://www.medrxiv.org/content/10.1101/2021.12.02.21267156v1
Anaphylaxis following Covid-19 vaccine in a patient with
cholinergic urticaria: https://pubmed.ncbi.nlm.nih.gov/33851711/
Anaphylaxis induced by CoronaVac COVID-19 vaccine: clinical
features and results of revaccination:
https://pubmed.ncbi.nlm.nih.gov/34675550/.
Anaphylaxis after Modern COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34734159/.
Association of self-reported history of high-risk allergy with
allergy symptoms after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34698847/
Sex differences in the incidence of anaphylaxis to LNP-mRNA
vaccines COVID-19: https://pubmed.ncbi.nlm.nih.gov/34020815/
Allergic reactions, including anaphylaxis, after receiving the
first dose of Pfizer-BioNTech COVID-19 vaccine – United States,
December 14 to 23, 2020: https://pubmed.ncbi.nlm.nih.gov/33641264/
Allergic reactions, including anaphylaxis, after receiving the
first dose of Modern COVID-19 vaccine – United States, December 21,
2020 to January 10, 2021: https://pubmed.ncbi.nlm.nih.gov/33641268/
Prolonged anaphylaxis to Pfizer 2019 coronavirus disease
vaccine: a case report and mechanism of action:
https://pubmed.ncbi.nlm.nih.gov/33834172/
Anaphylaxis reactions to Pfizer BNT162b2 vaccine: report of 3
cases of anaphylaxis following vaccination with Pfizer BNT162b2:
https://pubmed.ncbi.nlm.nih.gov/34579211/
Biphasic anaphylaxis after first dose of 2019 messenger RNA
coronavirus disease vaccine with positive polysorbate 80 skin test
result: https://pubmed.ncbi.nlm.nih.gov/34343674/
Acute myocardial infarction and myocarditis after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34586408/
Takotsubo syndrome after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34539938/.
Takotsubo cardiomyopathy after coronavirus 2019 vaccination in
patient on maintenance hemodialysis:
https://pubmed.ncbi.nlm.nih.gov/34731486/.
Premature myocardial infarction or side effect of COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/33824804/
Myocardial infarction, stroke, and pulmonary embolism after
BNT162b2 mRNA COVID-19 vaccine in persons aged 75 years or older:
https://pubmed.ncbi.nlm.nih.gov/34807248/
Kounis syndrome type 1 induced by inactivated SARS-COV-2
vaccine: https://pubmed.ncbi.nlm.nih.gov/34148772/
Acute myocardial infarction within 24 hours after COVID-19
vaccination: is Kounis syndrome the culprit:
https://pubmed.ncbi.nlm.nih.gov/34702550/
Deaths associated with the recently launched SARS-CoV-2
vaccination (Comirnaty®): https://pubmed.ncbi.nlm.nih.gov/33895650/
Deaths associated with recently launched SARS-CoV-2
vaccination: https://pubmed.ncbi.nlm.nih.gov/34425384/
A case of acute encephalopathy and non-ST-segment elevation
myocardial infarction after vaccination with mRNA-1273: possible
adverse effect: https://pubmed.ncbi.nlm.nih.gov/34703815/
COVID-19 vaccine-induced urticarial vasculitis:
https://pubmed.ncbi.nlm.nih.gov/34369046/.
ANCA-associated vasculitis after Pfizer-BioNTech COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34280507/.
New-onset leukocytoclastic vasculitis after COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34241833/
Cutaneous small vessel vasculitis after COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34529877/.
Outbreak of leukocytoclastic vasculitis after COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/33928638/
Leukocytoclastic vasculitis after exposure to COVID-19
vaccine: https://pubmed.ncbi.nlm.nih.gov/34836739/
Vasculitis and bursitis in [ 18 F] FDG-PET/CT after COVID-19
mRNA vaccine: post hoc ergo propter hoc?;
https://pubmed.ncbi.nlm.nih.gov/34495381/.
Cutaneous lymphocytic vasculitis after administration of
COVID-19 mRNA vaccine: https://pubmed.ncbi.nlm.nih.gov/34327795
Cutaneous leukocytoclastic vasculitis induced by Sinovac
COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34660867/.
Case report: ANCA-associated vasculitis presenting with
rhabdomyolysis and crescentic Pauci-Inmune glomerulonephritis after
vaccination with Pfizer-BioNTech COVID-19 mRNA:
https://pubmed.ncbi.nlm.nih.gov/34659268/
Reactivation of IgA vasculitis after vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34848431/
Varicella-zoster virus-related small-vessel vasculitis after
Pfizer-BioNTech COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34310759/.
Imaging in vascular medicine: leukocytoclastic vasculitis
after COVID-19 vaccine booster:
https://pubmed.ncbi.nlm.nih.gov/34720009/
A rare case of Henoch-Schönlein purpura after a case report
of COVID-19 vaccine: https://pubmed.ncbi.nlm.nih.gov/34518812/
Cutaneous vasculitis following COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34611627/.
Possible case of COVID-19 mRNA vaccine-induced small-vessel
vasculitis: https://pubmed.ncbi.nlm.nih.gov/34705320/.
IgA vasculitis following COVID-19 vaccination in an adult:
https://pubmed.ncbi.nlm.nih.gov/34779011/
Propylthiouracil-induced anti-neutrophil cytoplasmic
antibody-associated vasculitis following vaccination with COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34451967/
Coronavirus disease vaccine 2019 (COVID-19) in systemic lupus
erythematosus and neutrophil anti-cytoplasmic antibody-associated
vasculitis: https://pubmed.ncbi.nlm.nih.gov/33928459/
Reactivation of IgA vasculitis after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34250509/
Clinical and histopathologic spectrum of delayed adverse skin
reactions after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34292611/.
First description of immune complex vasculitis after COVID-19
vaccination with BNT162b2: case report:
https://pubmed.ncbi.nlm.nih.gov/34530771/.
Nephrotic syndrome and vasculitis after SARS-CoV-2 vaccine:
true association or circumstantial:
https://pubmed.ncbi.nlm.nih.gov/34245294/.
Occurrence of de novo cutaneous vasculitis after vaccination
against coronavirus disease (COVID-19):
https://pubmed.ncbi.nlm.nih.gov/34599716/.
Asymmetric cutaneous vasculitis after COVID-19 vaccination
with unusual preponderance of eosinophils:
https://pubmed.ncbi.nlm.nih.gov/34115904/.
Henoch-Schönlein purpura occurring after vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34247902/.
Henoch-Schönlein purpura following the first dose of COVID-19
viral vector vaccine: case report:
https://pubmed.ncbi.nlm.nih.gov/34696186/.
Granulomatous vasculitis after AstraZeneca anti-SARS-CoV-2
vaccine: https://pubmed.ncbi.nlm.nih.gov/34237323/.
Acute retinal necrosis due to varicella zoster virus
reactivation after vaccination with BNT162b2 COVID-19 mRNA:
https://pubmed.ncbi.nlm.nih.gov/34851795/.
A case of generalized Sweet’s syndrome with vasculitis
triggered by recent vaccination with COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34849386/
Small-vessel vasculitis following Oxford-AstraZeneca
vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/34310763/
Relapse of microscopic polyangiitis after COVID-19
vaccination: case report: https://pubmed.ncbi.nlm.nih.gov/34251683/.
Cutaneous vasculitis after severe acute respiratory syndrome
coronavirus 2 vaccine: https://pubmed.ncbi.nlm.nih.gov/34557622/.
Recurrent herpes zoster after COVID-19 vaccination in patients
with chronic urticaria on cyclosporine treatment – A report of 3
cases: https://pubmed.ncbi.nlm.nih.gov/34510694/
Leukocytoclastic vasculitis after coronavirus disease
vaccination 2019: https://pubmed.ncbi.nlm.nih.gov/34713472/803
Outbreaks of mixed cryoglobulinemia vasculitis after
vaccination against SARS-CoV-2:
https://pubmed.ncbi.nlm.nih.gov/34819272/
Cutaneous small-vessel vasculitis after vaccination with a
single dose of Janssen Ad26.COV2.S:
https://pubmed.ncbi.nlm.nih.gov/34337124/
Case of immunoglobulin A vasculitis after vaccination against
coronavirus disease 2019: https://pubmed.ncbi.nlm.nih.gov/34535924/
Rapid progression of angioimmunoblastic T-cell lymphoma after
BNT162b2 mRNA booster vaccination: case report:
https://www.frontiersin.org/articles/10.3389/fmed.2021.798095/
COVID-19 mRNA vaccination-induced lymphadenopathy mimics
lymphoma progression on FDG PET / CT:
https://pubmed.ncbi.nlm.nih.gov/33591026/
Lymphadenopathy in COVID-19 vaccine recipients: diagnostic
dilemma in oncology patients:
https://pubmed.ncbi.nlm.nih.gov/33625300/
Hypermetabolic lymphadenopathy after administration of
BNT162b2 mRNA vaccine Covid-19: incidence assessed by [ 18 F] FDG
PET-CT and relevance for study interpretation:
https://pubmed.ncbi.nlm.nih.gov/33774684/
Lymphadenopathy after COVID-19 vaccination: review of imaging
findings: https://pubmed.ncbi.nlm.nih.gov/33985872/
Evolution of bilateral hypermetabolic axillary hypermetabolic
lymphadenopathy on FDG PET/CT after 2-dose COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34735411/
Lymphadenopathy associated with COVID-19 vaccination on FDG
PET/CT: distinguishing features in adenovirus-vectored vaccine:
https://pubmed.ncbi.nlm.nih.gov/34115709/.
COVID-19 vaccination-induced lymphadenopathy in a specialized
breast imaging clinic in Israel: analysis of 163 cases:
https://pubmed.ncbi.nlm.nih.gov/34257025/.
COVID-19 vaccine-related axillary lymphadenopathy in breast
cancer patients: case series with literature review:
https://pubmed.ncbi.nlm.nih.gov/34836672/.
Coronavirus disease vaccine 2019 mimics lymph node metastases
in patients undergoing skin cancer follow-up: a single-center study:
https://pubmed.ncbi.nlm.nih.gov/34280870/
COVID-19 post-vaccination lymphadenopathy: report of
fine-needle aspiration biopsy cytologic findings:
https://pubmed.ncbi.nlm.nih.gov/34432391/
Regional lymphadenopathy after COVID-19 vaccination: review of
the literature and considerations for patient management in breast
cancer care: https://pubmed.ncbi.nlm.nih.gov/34731748/
Subclinical axillary lymphadenopathy associated with COVID-19
vaccination on screening mammography:
https://pubmed.ncbi.nlm.nih.gov/34906409/
Adverse events of COVID injection that may occur in
children.Acute-onset supraclavicular lymphadenopathy coincident with
intramuscular mRNA vaccination against COVID-19 may be related to the
injection technique of the vaccine, Spain, January and February 2021:
https://pubmed.ncbi.nlm.nih.gov/33706861/
Supraclavicular lymphadenopathy after COVID-19 vaccination in
Korea: serial follow-up by ultrasonography:
https://pubmed.ncbi.nlm.nih.gov/34116295/
Oxford-AstraZeneca COVID-19 vaccination induced
lymphadenopathy on [18F] choline PET / CT, not just an FDG finding:
https://pubmed.ncbi.nlm.nih.gov/33661328/
Biphasic anaphylaxis after exposure to the first dose of
Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34050949/
Axillary adenopathy associated with COVID-19 vaccination:
imaging findings and follow-up recommendations in 23 women:
https://pubmed.ncbi.nlm.nih.gov/33624520/
A case of cervical lymphadenopathy following COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34141500/
Unique imaging findings of neurologic phantosmia after
Pfizer-BioNtech COVID-19 vaccination: a case report:
https://pubmed.ncbi.nlm.nih.gov/34096896/
Thrombotic adverse events reported for Moderna, Pfizer, and
Oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and
clinical outcomes in the EudraVigilance database:
https://pubmed.ncbi.nlm.nih.gov/34835256/
Unilateral lymphadenopathy after COVID-19 vaccination: a
practical management plan for radiologists of all specialties:
https://pubmed.ncbi.nlm.nih.gov/33713605/
Unilateral axillary adenopathy in the setting of COVID-19
vaccination: follow-up: https://pubmed.ncbi.nlm.nih.gov/34298342/
A systematic review of cases of CNS demyelination following
COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34839149/
Supraclavicular lymphadenopathy after COVID-19 vaccination: an
increasing presentation in the two-week wait neck lump clinic:
https://pubmed.ncbi.nlm.nih.gov/33685772/
COVID-19 vaccine-related axillary and cervical lymphadenopathy
in patients with current or previous breast cancer and other
malignancies: cross-sectional imaging findings on MRI, CT and PET-CT:
https://pubmed.ncbi.nlm.nih.gov/34719892/
Adenopathy after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33625299/.
Incidence of axillary adenopathy on breast imaging after
vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34292295/.
COVID-19 vaccination and lower cervical lymphadenopathy in
two-week neck lump clinic: a follow-up audit:
https://pubmed.ncbi.nlm.nih.gov/33947605/.
Cervical lymphadenopathy after coronavirus disease vaccination
2019: clinical features and implications for head and neck cancer
services: https://pubmed.ncbi.nlm.nih.gov/34526175/
Lymphadenopathy associated with the COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33786231/
Evolution of lymphadenopathy on PET/MRI after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33625301/.
Autoimmune hepatitis triggered by SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34332438/.
New-onset nephrotic syndrome after Janssen COVID-19
vaccination: case report and literature review:
https://pubmed.ncbi.nlm.nih.gov/34342187/.
Massive cervical lymphadenopathy following vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34601889/
ANCA glomerulonephritis following Modern COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34081948/
Extensive longitudinal transverse myelitis following
AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34507942/.
Systemic capillary extravasation syndrome after vaccination
with ChAdOx1 nCOV-19 (Oxford-AstraZeneca):
https://pubmed.ncbi.nlm.nih.gov/34362727/
Unilateral axillary lymphadenopathy related to COVID-19
vaccine: pattern on screening breast MRI allowing benign evaluation:
https://pubmed.ncbi.nlm.nih.gov/34325221/
Axillary lymphadenopathy in patients with recent Covid-19
vaccination: a new diagnostic dilemma:
https://pubmed.ncbi.nlm.nih.gov/34825530/.
Minimal change disease and acute kidney injury after
Pfizer-BioNTech COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34000278/
COVID-19 vaccine-induced unilateral axillary adenopathy:
follow-up evaluation in the USA:
https://pubmed.ncbi.nlm.nih.gov/34655312/.
Gastroparesis after Pfizer-BioNTech COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34187985/.
Acute-onset supraclavicular lymphadenopathy coincident with
intramuscular mRNA vaccination against COVID-19 may be related to the
injection technique of the vaccine, Spain, January and February 2021:
https://pubmed.ncbi.nlm.nih.gov/33706861/
Supraclavicular lymphadenopathy after COVID-19 vaccination in
Korea: serial follow-up by ultrasonography:
https://pubmed.ncbi.nlm.nih.gov/34116295/
Oxford-AstraZeneca COVID-19 vaccination induced
lymphadenopathy on [18F] choline PET / CT, not just an FDG finding:
https://pubmed.ncbi.nlm.nih.gov/33661328/
Biphasic anaphylaxis after exposure to the first dose of
Pfizer-BioNTech COVID-19 mRNA vaccine COVID-19:
https://pubmed.ncbi.nlm.nih.gov/34050949/
Axillary adenopathy associated with COVID-19 vaccination:
imaging findings and follow-up recommendations in 23 women:
https://pubmed.ncbi.nlm.nih.gov/33624520/
A case of cervical lymphadenopathy following COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/34141500/
Unique imaging findings of neurologic phantosmia after
Pfizer-BioNtech COVID-19 vaccination: a case report:
https://pubmed.ncbi.nlm.nih.gov/34096896/
Thrombotic adverse events reported for Moderna, Pfizer, and
Oxford-AstraZeneca COVID-19 vaccines: comparison of occurrence and
clinical outcomes in the EudraVigilance database:
https://pubmed.ncbi.nlm.nih.gov/34835256/
Unilateral lymphadenopathy after COVID-19 vaccination: a
practical management plan for radiologists of all specialties:
https://pubmed.ncbi.nlm.nih.gov/33713605/
Unilateral axillary adenopathy in the setting of COVID-19
vaccination: follow-up: https://pubmed.ncbi.nlm.nih.gov/34298342/
A systematic review of cases of CNS demyelination following
COVID-19 vaccination: https://pubmed.ncbi.nlm.nih.gov/34839149/
Supraclavicular lymphadenopathy after COVID-19 vaccination: an
increasing presentation in the two-week wait neck lump clinic:
https://pubmed.ncbi.nlm.nih.gov/33685772/
COVID-19 vaccine-related axillary and cervical lymphadenopathy
in patients with current or previous breast cancer and other
malignancies: cross-sectional imaging findings on MRI, CT and PET-CT:
https://pubmed.ncbi.nlm.nih.gov/34719892/
Adenopathy after COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/33625299/.
Incidence of axillary adenopathy on breast imaging after
vaccination with COVID-19: https://pubmed.ncbi.nlm.nih.gov/34292295/.
COVID-19 vaccination and lower cervical lymphadenopathy in
two-week neck lump clinic: a follow-up audit:
https://pubmed.ncbi.nlm.nih.gov/33947605/.
Cervical lymphadenopathy after coronavirus disease vaccination
2019: clinical features and implications for head and neck cancer
services: https://pubmed.ncbi.nlm.nih.gov/34526175/
Lymphadenopathy associated with the COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/33786231/
Evolution of lymphadenopathy on PET/MRI after COVID-19
vaccination: https://pubmed.ncbi.nlm.nih.gov/33625301/.
Autoimmune hepatitis triggered by SARS-CoV-2 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34332438/.
New-onset nephrotic syndrome after Janssen COVID-19
vaccination: case report and literature review:
https://pubmed.ncbi.nlm.nih.gov/34342187/.
Massive cervical lymphadenopathy following vaccination with
COVID-19: https://pubmed.ncbi.nlm.nih.gov/34601889/
ANCA glomerulonephritis following Modern COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34081948/
Extensive longitudinal transverse myelitis following
AstraZeneca COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34507942/.
Systemic capillary extravasation syndrome after vaccination
with ChAdOx1 nCOV-19 (Oxford-AstraZeneca):
https://pubmed.ncbi.nlm.nih.gov/34362727/
Unilateral axillary lymphadenopathy related to COVID-19
vaccine: pattern on screening breast MRI allowing benign evaluation:
https://pubmed.ncbi.nlm.nih.gov/34325221/
Axillary lymphadenopathy in patients with recent Covid-19
vaccination: a new diagnostic dilemma:
https://pubmed.ncbi.nlm.nih.gov/34825530/.
Minimal change disease and acute kidney injury after
Pfizer-BioNTech COVID-19 vaccine:
https://pubmed.ncbi.nlm.nih.gov/34000278/
COVID-19 vaccine-induced unilateral axillary adenopathy:
follow-up evaluation in the USA:
https://pubmed.ncbi.nlm.nih.gov/34655312/.
Gastroparesis after Pfizer-BioNTech COVID-19 vaccination:
https://pubmed.ncbi.nlm.nih.gov/34187985/.
Abbate, A., Gavin, J., Madanchi, N., Kim, C., Shah, P. R.,
Klein, K., . . . Danielides, S. (2021). Fulminant myocarditis and
systemic hyperinflammation temporally associated with BNT162b2 mRNA
COVID-19 vaccination in two patients. Int J Cardiol, 340, 119-121.
doi:10.1016/j.ijcard.2021.08.018.
https://www.ncbi.nlm.nih.gov/pubmed/34416319
Abu Mouch, S., Roguin, A., Hellou, E., Ishai, A., Shoshan, U.,
Mahamid, L., . . . Berar Yanay, N. (2021). Myocarditis following
COVID-19 mRNA vaccination. Vaccine, 39(29), 3790-3793.
doi:10.1016/j.vaccine.2021.05.087.
https://www.ncbi.nlm.nih.gov/pubmed/34092429
Albert, E., Aurigemma, G., Saucedo, J., & Gerson, D. S.
(2021). Myocarditis following COVID-19 vaccination. Radiol Case Rep,
16(8), 2142-2145. doi:10.1016/j.radcr.2021.05.033.
https://www.ncbi.nlm.nih.gov/pubmed/34025885
Aye, Y. N., Mai, A. S., Zhang, A., Lim, O. Z. H., Lin, N., Ng,
C. H., . . . Chew, N. W. S. (2021). Acute Myocardial Infarction and
Myocarditis following COVID-19 Vaccination. QJM.
doi:10.1093/qjmed/hcab252.
https://www.ncbi.nlm.nih.gov/pubmed/34586408
Azir, M., Inman, B., Webb, J., & Tannenbaum, L. (2021).
STEMI Mimic: Focal Myocarditis in an Adolescent Patient After mRNA
COVID-19 Vaccine. J Emerg Med, 61(6), e129-e132.
doi:10.1016/j.jemermed.2021.09.017.
https://www.ncbi.nlm.nih.gov/pubmed/34756746
Barda, N., Dagan, N., Ben-Shlomo, Y., Kepten, E., Waxman, J.,
Ohana, R., . . . Balicer, R. D. (2021). Safety of the BNT162b2 mRNA
Covid-19 Vaccine in a Nationwide Setting. N Engl J Med, 385(12),
1078-1090. doi:10.1056/NEJMoa2110475.
https://www.ncbi.nlm.nih.gov/pubmed/34432976
Bhandari, M., Pradhan, A., Vishwakarma, P., & Sethi, R.
(2021). Coronavirus and cardiovascular manifestations- getting to the
heart of the matter. World J Cardiol, 13(10), 556-565.
doi:10.4330/wjc.v13.i10.556.
https://www.ncbi.nlm.nih.gov/pubmed/34754400
Bozkurt, B., Kamat, I., & Hotez, P. J. (2021). Myocarditis
With COVID-19 mRNA Vaccines. Circulation, 144(6), 471-484.
doi:10.1161/CIRCULATIONAHA.121.056135.
https://www.ncbi.nlm.nih.gov/pubmed/34281357
Buchhorn, R., Meyer, C., Schulze-Forster, K., Junker, J., &
Heidecke, H. (2021). Autoantibody Release in Children after Corona
Virus mRNA Vaccination: A Risk Factor of Multisystem Inflammatory
Syndrome? Vaccines (Basel), 9(11). doi:10.3390/vaccines9111353.
https://www.ncbi.nlm.nih.gov/pubmed/34835284
Calcaterra, G., Bassareo, P. P., Barilla, F., Romeo, F., &
Mehta, J. L. (2022). Concerning the unexpected prothrombotic state
following some coronavirus disease 2019 vaccines. J Cardiovasc Med
(Hagerstown), 23(2), 71-74. doi:10.2459/JCM.0000000000001232.
https://www.ncbi.nlm.nih.gov/pubmed/34366403
Calcaterra, G., Mehta, J. L., de Gregorio, C., Butera, G.,
Neroni, P., Fanos, V., & Bassareo, P. P. (2021). COVID 19 Vaccine
for Adolescents. Concern about Myocarditis and Pericarditis. Pediatr
Rep, 13(3), 530-533. doi:10.3390/pediatric13030061.
https://www.ncbi.nlm.nih.gov/pubmed/34564344
Chai, Q., Nygaard, U., Schmidt, R. C., Zaremba, T., Moller, A.
M., & Thorvig, C. M. (2022). Multisystem inflammatory syndrome in
a male adolescent after his second Pfizer-BioNTech COVID-19 vaccine.
Acta Paediatr, 111(1), 125-127. doi:10.1111/apa.16141.
https://www.ncbi.nlm.nih.gov/pubmed/34617315
Chamling, B., Vehof, V., Drakos, S., Weil, M., Stalling, P.,
Vahlhaus, C., . . . Yilmaz, A. (2021). Occurrence of acute
infarct-like myocarditis following COVID-19 vaccination: just an
accidental co-incidence or rather vaccination-associated autoimmune
myocarditis? Clin Res Cardiol, 110(11), 1850-1854.
doi:10.1007/s00392-021-01916-w.
https://www.ncbi.nlm.nih.gov/pubmed/34333695
Chang, J. C., & Hawley, H. B. (2021). Vaccine-Associated
Thrombocytopenia and Thrombosis: Venous Endotheliopathy Leading to
Venous Combined Micro-Macrothrombosis. Medicina (Kaunas), 57(11).
doi:10.3390/medicina57111163.
https://www.ncbi.nlm.nih.gov/pubmed/34833382
Chelala, L., Jeudy, J., Hossain, R., Rosenthal, G., Pietris,
N., & White, C. (2021). Cardiac MRI Findings of Myocarditis After
COVID-19 mRNA Vaccination in Adolescents. AJR Am J Roentgenol.
doi:10.2214/AJR.21.26853.
https://www.ncbi.nlm.nih.gov/pubmed/34704459
Choi, S., Lee, S., Seo, J. W., Kim, M. J., Jeon, Y. H., Park,
J. H., . . . Yeo, N. S. (2021). Myocarditis-induced Sudden Death
after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report
Focusing on Histopathological Findings. J Korean Med Sci, 36(40),
e286. doi:10.3346/jkms.2021.36.e286.
https://www.ncbi.nlm.nih.gov/pubmed/34664804
Chouchana, L., Blet, A., Al-Khalaf, M., Kafil, T. S., Nair,
G., Robblee, J., . . . Liu, P. P. (2021). Features of Inflammatory
Heart Reactions Following mRNA COVID-19 Vaccination at a Global
Level. Clin Pharmacol Ther. doi:10.1002/cpt.2499.
https://www.ncbi.nlm.nih.gov/pubmed/34860360
Chua, G. T., Kwan, M. Y. W., Chui, C. S. L., Smith, R. D.,
Cheung, E. C., Tian, T., . . . Ip, P. (2021). Epidemiology of Acute
Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty
Vaccination. Clin Infect Dis. doi:10.1093/cid/ciab989.
https://www.ncbi.nlm.nih.gov/pubmed/34849657
Clarke, R., & Ioannou, A. (2021). Should T2 mapping be
used in cases of recurrent myocarditis to differentiate between the
acute inflammation and chronic scar? J Pediatr.
doi:10.1016/j.jpeds.2021.12.026.
https://www.ncbi.nlm.nih.gov/pubmed/34933012
Colaneri, M., De Filippo, M., Licari, A., Marseglia, A.,
Maiocchi, L., Ricciardi, A., . . . Bruno, R. (2021). COVID
vaccination and asthma exacerbation: might there be a link? Int J
Infect Dis, 112, 243-246. doi:10.1016/j.ijid.2021.09.026.
https://www.ncbi.nlm.nih.gov/pubmed/34547487
Das, B. B., Kohli, U., Ramachandran, P., Nguyen, H. H., Greil,
G., Hussain, T., . . . Khan, D. (2021). Myopericarditis after
messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12
to 18 Years of Age. J Pediatr, 238, 26-32 e21.
doi:10.1016/j.jpeds.2021.07.044.
https://www.ncbi.nlm.nih.gov/pubmed/34339728
Das, B. B., Moskowitz, W. B., Taylor, M. B., & Palmer, A.
(2021). Myocarditis and Pericarditis Following mRNA COVID-19
Vaccination: What Do We Know So Far? Children (Basel), 8(7).
doi:10.3390/children8070607.
https://www.ncbi.nlm.nih.gov/pubmed/34356586
Deb, A., Abdelmalek, J., Iwuji, K., & Nugent, K. (2021).
Acute Myocardial Injury Following COVID-19 Vaccination: A Case Report
and Review of Current Evidence from Vaccine Adverse Events Reporting
System Database. J Prim Care Community Health, 12, 21501327211029230.
doi:10.1177/21501327211029230.
https://www.ncbi.nlm.nih.gov/pubmed/34219532
Dickey, J. B., Albert, E., Badr, M., Laraja, K. M., Sena, L.
M., Gerson, D. S., . . . Aurigemma, G. P. (2021). A Series of
Patients With Myocarditis Following SARS-CoV-2 Vaccination With
mRNA-1279 and BNT162b2. JACC Cardiovasc Imaging, 14(9), 1862-1863.
doi:10.1016/j.jcmg.2021.06.003.
https://www.ncbi.nlm.nih.gov/pubmed/34246585
Dimopoulou, D., Spyridis, N., Vartzelis, G., Tsolia, M. N., &
Maritsi, D. N. (2021). Safety and tolerability of the COVID-19
mRNA-vaccine in adolescents with juvenile idiopathic arthritis on
treatment with TNF-inhibitors. Arthritis Rheumatol.
doi:10.1002/art.41977. https://www.ncbi.nlm.nih.gov/pubmed/34492161
Dimopoulou, D., Vartzelis, G., Dasoula, F., Tsolia, M., &
Maritsi, D. (2021). Immunogenicity of the COVID-19 mRNA vaccine in
adolescents with juvenile idiopathic arthritis on treatment with TNF
inhibitors. Ann Rheum Dis. doi:10.1136/annrheumdis-2021-221607.
https://www.ncbi.nlm.nih.gov/pubmed/34844930
Ehrlich, P., Klingel, K., Ohlmann-Knafo, S., Huttinger, S.,
Sood, N., Pickuth, D., & Kindermann, M. (2021). Biopsy-proven
lymphocytic myocarditis following first mRNA COVID-19 vaccination in
a 40-year-old male: case report. Clin Res Cardiol, 110(11),
1855-1859. doi:10.1007/s00392-021-01936-6.
https://www.ncbi.nlm.nih.gov/pubmed/34487236
El Sahly, H. M., Baden, L. R., Essink, B., Doblecki-Lewis, S.,
Martin, J. M., Anderson, E. J., . . . Group, C. S. (2021). Efficacy
of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N
Engl J Med, 385(19), 1774-1785. doi:10.1056/NEJMoa2113017.
https://www.ncbi.nlm.nih.gov/pubmed/34551225
Facetti, S., Giraldi, M., Vecchi, A. L., Rogiani, S., &
Nassiacos, D. (2021). [Acute myocarditis in a young adult two days
after Pfizer vaccination]. G Ital Cardiol (Rome), 22(11), 891-893.
doi:10.1714/3689.36746. https://www.ncbi.nlm.nih.gov/pubmed/34709227
Fazlollahi, A., Zahmatyar, M., Noori, M., Nejadghaderi, S. A.,
Sullman, M. J. M., Shekarriz-Foumani, R., . . . Safiri, S. (2021).
Cardiac complications following mRNA COVID-19 vaccines: A systematic
review of case reports and case series. Rev Med Virol, e2318.
doi:10.1002/rmv.2318. https://www.ncbi.nlm.nih.gov/pubmed/34921468
Fazolo, T., Lima, K., Fontoura, J. C., de Souza, P. O.,
Hilario, G., Zorzetto, R., . . . Bonorino, C. (2021). Pediatric
COVID-19 patients in South Brazil show abundant viral mRNA and strong
specific anti-viral responses. Nat Commun, 12(1), 6844.
doi:10.1038/s41467-021-27120-y.
https://www.ncbi.nlm.nih.gov/pubmed/34824230
Fikenzer, S., & Laufs, U. (2021). Correction to: Response
to Letter to the editors referring to Fikenzer, S., Uhe, T., Lavall,
D., Rudolph, U., Falz, R., Busse, M., Hepp, P., & Laufs, U.
(2020). Effects of surgical and FFP2/N95 face masks on
cardiopulmonary exercise capacity. Clinical research in cardiology:
official journal of the German Cardiac Society, 1-9. Advance online
publication. https://doi.org/10.1007/s00392-020-01704-y. Clin Res
Cardiol, 110(8), 1352. doi:10.1007/s00392-021-01896-x.
https://www.ncbi.nlm.nih.gov/pubmed/34170372
Foltran, D., Delmas, C., Flumian, C., De Paoli, P., Salvo, F.,
Gautier, S., . . . Montastruc, F. (2021). Myocarditis and
Pericarditis in Adolescents after First and Second doses of mRNA
COVID-19 Vaccines. Eur Heart J Qual Care Clin Outcomes.
doi:10.1093/ehjqcco/qcab090.
https://www.ncbi.nlm.nih.gov/pubmed/34849667
Forgacs, D., Jang, H., Abreu, R. B., Hanley, H. B., Gattiker,
J. L., Jefferson, A. M., & Ross, T. M. (2021). SARS-CoV-2 mRNA
Vaccines Elicit Different Responses in Immunologically Naive and
Pre-Immune Humans. Front Immunol, 12, 728021.
doi:10.3389/fimmu.2021.728021.
https://www.ncbi.nlm.nih.gov/pubmed/34646267
Furer, V., Eviatar, T., Zisman, D., Peleg, H., Paran, D.,
Levartovsky, D., . . . Elkayam, O. (2021). Immunogenicity and safety
of the BNT162b2 mRNA COVID-19 vaccine in adult patients with
autoimmune inflammatory rheumatic diseases and in the general
population: a multicentre study. Ann Rheum Dis, 80(10), 1330-1338.
doi:10.1136/annrheumdis-2021-220647.
https://www.ncbi.nlm.nih.gov/pubmed/34127481
Galindo, R., Chow, H., & Rongkavilit, C. (2021). COVID-19
in Children: Clinical Manifestations and Pharmacologic Interventions
Including Vaccine Trials. Pediatr Clin North Am, 68(5), 961-976.
doi:10.1016/j.pcl.2021.05.004.
https://www.ncbi.nlm.nih.gov/pubmed/34538306
Gargano, J. W., Wallace, M., Hadler, S. C., Langley, G., Su,
J. R., Oster, M. E., . . . Oliver, S. E. (2021). Use of mRNA COVID-19
Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update
from the Advisory Committee on Immunization Practices – United
States, June 2021. MMWR Morb Mortal Wkly Rep, 70(27), 977-982.
doi:10.15585/mmwr.mm7027e2.
https://www.ncbi.nlm.nih.gov/pubmed/34237049
Gatti, M., Raschi, E., Moretti, U., Ardizzoni, A., Poluzzi,
E., & Diemberger, I. (2021). Influenza Vaccination and
Myo-Pericarditis in Patients Receiving Immune Checkpoint Inhibitors:
Investigating the Likelihood of Interaction through the Vaccine
Adverse Event Reporting System and VigiBase. Vaccines (Basel), 9(1).
doi:10.3390/vaccines9010019.
https://www.ncbi.nlm.nih.gov/pubmed/33406694
Gautam, N., Saluja, P., Fudim, M., Jambhekar, K., Pandey, T.,
& Al’Aref, S. (2021). A Late Presentation of COVID-19
Vaccine-Induced Myocarditis. Cureus, 13(9), e17890.
doi:10.7759/cureus.17890.
https://www.ncbi.nlm.nih.gov/pubmed/34660088
Gellad, W. F. (2021). Myocarditis after vaccination against
covid-19. BMJ, 375, n3090. doi:10.1136/bmj.n3090.
https://www.ncbi.nlm.nih.gov/pubmed/34916217
Greenhawt, M., Abrams, E. M., Shaker, M., Chu, D. K., Khan,
D., Akin, C., . . . Golden, D. B. K. (2021). The Risk of Allergic
Reaction to SARS-CoV-2 Vaccines and Recommended Evaluation and
Management: A Systematic Review, Meta-Analysis, GRADE Assessment, and
International Consensus Approach. J Allergy Clin Immunol Pract,
9(10), 3546-3567. doi:10.1016/j.jaip.2021.06.006.
https://www.ncbi.nlm.nih.gov/pubmed/34153517
Haaf, P., Kuster, G. M., Mueller, C., Berger, C. T., Monney,
P., Burger, P., . . . Tanner, F. C. (2021). The very low risk of
myocarditis and pericarditis after mRNA COVID-19 vaccination should
not discourage vaccination. Swiss Med Wkly, 151, w30087.
doi:10.4414/smw.2021.w30087.
https://www.ncbi.nlm.nih.gov/pubmed/34668687
Hasnie, A. A., Hasnie, U. A., Patel, N., Aziz, M. U., Xie, M.,
Lloyd, S. G., & Prabhu, S. D. (2021). Perimyocarditis following
first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy
young male: a case report. BMC Cardiovasc Disord, 21(1), 375.
doi:10.1186/s12872-021-02183-3.
https://www.ncbi.nlm.nih.gov/pubmed/34348657
Hause, A. M., Gee, J., Baggs, J., Abara, W. E., Marquez, P.,
Thompson, D., . . . Shay, D. K. (2021). COVID-19 Vaccine Safety in
Adolescents Aged 12-17 Years – United States, December 14,
2020-July 16, 2021. MMWR Morb Mortal Wkly Rep, 70(31), 1053-1058.
doi:10.15585/mmwr.mm7031e1.
https://www.ncbi.nlm.nih.gov/pubmed/34351881
Helms, J. M., Ansteatt, K. T., Roberts, J. C., Kamatam, S.,
Foong, K. S., Labayog, J. S., & Tarantino, M. D. (2021). Severe,
Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2
Vaccine. J Blood Med, 12, 221-224. doi:10.2147/JBM.S307047.
https://www.ncbi.nlm.nih.gov/pubmed/33854395
Hippisley-Cox, J., Patone, M., Mei, X. W., Saatci, D., Dixon,
S., Khunti, K., . . . Coupland, C. A. C. (2021). Risk of
thrombocytopenia and thromboembolism after covid-19 vaccination and
SARS-CoV-2 positive testing: self-controlled case series study. BMJ,
374, n1931. doi:10.1136/bmj.n1931.
https://www.ncbi.nlm.nih.gov/pubmed/34446426
Ho, J. S., Sia, C. H., Ngiam, J. N., Loh, P. H., Chew, N. W.,
Kong, W. K., & Poh, K. K. (2021). A review of COVID-19
vaccination and the reported cardiac manifestations. Singapore Med J.
doi:10.11622/smedj.2021210.
https://www.ncbi.nlm.nih.gov/pubmed/34808708
Iguchi, T., Umeda, H., Kojima, M., Kanno, Y., Tanaka, Y.,
Kinoshita, N., & Sato, D. (2021). Cumulative Adverse Event
Reporting of Anaphylaxis After mRNA COVID-19 Vaccine
(Pfizer-BioNTech) Injections in Japan: The First-Month Report. Drug
Saf, 44(11), 1209-1214. doi:10.1007/s40264-021-01104-9.
https://www.ncbi.nlm.nih.gov/pubmed/34347278
In brief: Myocarditis with the Pfizer/BioNTech and Moderna
COVID-19 vaccines. (2021). Med Lett Drugs Ther, 63(1629), e9.
Retrieved from
https://www.ncbi.nlm.nih.gov/pubmed/34544112https://www.ncbi.nlm.nih.gov/pubmed/3454412
Ioannou, A. (2021a). Myocarditis should be considered in those
with a troponin rise and unobstructed coronary arteries following
Pfizer-BioNTech COVID-19 vaccination. QJM. doi:10.1093/qjmed/hcab231.
https://www.ncbi.nlm.nih.gov/pubmed/34463755
Ioannou, A. (2021b). T2 mapping should be utilised in cases of
suspected myocarditis to confirm an acute inflammatory process. QJM.
doi:10.1093/qjmed/hcab326.
https://www.ncbi.nlm.nih.gov/pubmed/34931681
Isaak, A., Feisst, A., & Luetkens, J. A. (2021).
Myocarditis Following COVID-19 Vaccination. Radiology, 301(1),
E378-E379. doi:10.1148/radiol.2021211766.
https://www.ncbi.nlm.nih.gov/pubmed/34342500
Istampoulouoglou, I., Dimitriou, G., Spani, S., Christ, A.,
Zimmermanns, B., Koechlin, S., . . . Leuppi-Taegtmeyer, A. B. (2021).
Myocarditis and pericarditis in association with COVID-19
mRNA-vaccination: cases from a regional pharmacovigilance centre.
Glob Cardiol Sci Pract, 2021(3), e202118. doi:10.21542/gcsp.2021.18.
https://www.ncbi.nlm.nih.gov/pubmed/34805376
Jaafar, R., Boschi, C., Aherfi, S., Bancod, A., Le Bideau, M.,
Edouard, S., . . . La Scola, B. (2021). High Individual Heterogeneity
of Neutralizing Activities against the Original Strain and Nine
Different Variants of SARS-CoV-2. Viruses, 13(11).
doi:10.3390/v13112177. https://www.ncbi.nlm.nih.gov/pubmed/34834983
Jain, S. S., Steele, J. M., Fonseca, B., Huang, S., Shah, S.,
Maskatia, S. A., . . . Grosse-Wortmann, L. (2021). COVID-19
Vaccination-Associated Myocarditis in Adolescents. Pediatrics,
148(5). doi:10.1542/peds.2021-053427.
https://www.ncbi.nlm.nih.gov/pubmed/34389692
Jhaveri, R., Adler-Shohet, F. C., Blyth, C. C., Chiotos, K.,
Gerber, J. S., Green, M., . . . Zaoutis, T. (2021). Weighing the
Risks of Perimyocarditis With the Benefits of SARS-CoV-2 mRNA
Vaccination in Adolescents. J Pediatric Infect Dis Soc, 10(10),
937-939. doi:10.1093/jpids/piab061.
https://www.ncbi.nlm.nih.gov/pubmed/34270752
Kaneta, K., Yokoi, K., Jojima, K., Kotooka, N., & Node, K.
(2021). Young Male With Myocarditis Following mRNA-1273 Vaccination
Against Coronavirus Disease-2019 (COVID-19). Circ J.
doi:10.1253/circj.CJ-21-0818.
https://www.ncbi.nlm.nih.gov/pubmed/34744118
Kaul, R., Sreenivasan, J., Goel, A., Malik, A., Bandyopadhyay,
D., Jin, C., . . . Panza, J. A. (2021). Myocarditis following
COVID-19 vaccination. Int J Cardiol Heart Vasc, 36, 100872.
doi:10.1016/j.ijcha.2021.100872.
https://www.ncbi.nlm.nih.gov/pubmed/34568540
Khogali, F., & Abdelrahman, R. (2021). Unusual
Presentation of Acute Perimyocarditis Following SARS-COV-2 mRNA-1237
Moderna Vaccination. Cureus, 13(7), e16590. doi:10.7759/cureus.16590.
https://www.ncbi.nlm.nih.gov/pubmed/34447639
Kim, H. W., Jenista, E. R., Wendell, D. C., Azevedo, C. F.,
Campbell, M. J., Darty, S. N., . . . Kim, R. J. (2021). Patients With
Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol,
6(10), 1196-1201. doi:10.1001/jamacardio.2021.2828.
https://www.ncbi.nlm.nih.gov/pubmed/34185046
Kim, I. C., Kim, H., Lee, H. J., Kim, J. Y., & Kim, J. Y.
(2021). Cardiac Imaging of Acute Myocarditis Following COVID-19 mRNA
Vaccination. J Korean Med Sci, 36(32), e229.
doi:10.3346/jkms.2021.36.e229.
https://www.ncbi.nlm.nih.gov/pubmed/34402228
King, W. W., Petersen, M. R., Matar, R. M., Budweg, J. B.,
Cuervo Pardo, L., & Petersen, J. W. (2021). Myocarditis following
mRNA vaccination against SARS-CoV-2, a case series. Am Heart J Plus,
8, 100042. doi:10.1016/j.ahjo.2021.100042.
https://www.ncbi.nlm.nih.gov/pubmed/34396358
Klein, N. P., Lewis, N., Goddard, K., Fireman, B., Zerbo, O.,
Hanson, K. E., . . . Weintraub, E. S. (2021). Surveillance for
Adverse Events After COVID-19 mRNA Vaccination. JAMA, 326(14),
1390-1399. doi:10.1001/jama.2021.15072.
https://www.ncbi.nlm.nih.gov/pubmed/34477808
Klimek, L., Bergmann, K. C., Brehler, R., Pfutzner, W.,
Zuberbier, T., Hartmann, K., . . . Worm, M. (2021). Practical
handling of allergic reactions to COVID-19 vaccines: A position paper
from German and Austrian Allergy Societies AeDA, DGAKI, GPA and OGAI.
Allergo J Int, 1-17. doi:10.1007/s40629-021-00165-7.
https://www.ncbi.nlm.nih.gov/pubmed/33898162
Klimek, L., Novak, N., Hamelmann, E., Werfel, T., Wagenmann,
M., Taube, C., . . . Worm, M. (2021). Severe allergic reactions after
COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great
Britain and USA: Position statement of the German Allergy Societies:
Medical Association of German Allergologists (AeDA), German Society
for Allergology and Clinical Immunology (DGAKI) and Society for
Pediatric Allergology and Environmental Medicine (GPA). Allergo J
Int, 30(2), 51-55. doi:10.1007/s40629-020-00160-4.
https://www.ncbi.nlm.nih.gov/pubmed/33643776
Kohli, U., Desai, L., Chowdhury, D., Harahsheh, A. S., Yonts,
A. B., Ansong, A., . . . Ang, J. Y. (2021). mRNA Coronavirus-19
Vaccine-Associated Myopericarditis in Adolescents: A Survey Study. J
Pediatr. doi:10.1016/j.jpeds.2021.12.025.
https://www.ncbi.nlm.nih.gov/pubmed/34952008
Kostoff, R. N., Calina, D., Kanduc, D., Briggs, M. B.,
Vlachoyiannopoulos, P., Svistunov, A. A., & Tsatsakis, A.
(2021a). Erratum to „Why are we vaccinating children against
COVID-19?” [Toxicol. Rep. 8C (2021) 1665-1684 / 1193]. Toxicol Rep,
8, 1981. doi:10.1016/j.toxrep.2021.10.003.
https://www.ncbi.nlm.nih.gov/pubmed/34642628
Kostoff, R. N., Calina, D., Kanduc, D., Briggs, M. B.,
Vlachoyiannopoulos, P., Svistunov, A. A., & Tsatsakis, A.
(2021b). Why are we vaccinating children against COVID-19? Toxicol
Rep, 8, 1665-1684. doi:10.1016/j.toxrep.2021.08.010.
https://www.ncbi.nlm.nih.gov/pubmed/34540594
Kremsner, P. G., Mann, P., Kroidl, A., Leroux-Roels, I.,
Schindler, C., Gabor, J. J., . . . Group, C.-N.-S. (2021). Safety and
immunogenicity of an mRNA-lipid nanoparticle vaccine candidate
against SARS-CoV-2 : A phase 1 randomized clinical trial. Wien Klin
Wochenschr, 133(17-18), 931-941. doi:10.1007/s00508-021-01922-y.
https://www.ncbi.nlm.nih.gov/pubmed/34378087
Kustin, T., Harel, N., Finkel, U., Perchik, S., Harari, S.,
Tahor, M., . . . Stern, A. (2021). Evidence for increased
breakthrough rates of SARS-CoV-2 variants of concern in
BNT162b2-mRNA-vaccinated individuals. Nat Med, 27(8), 1379-1384.
doi:10.1038/s41591-021-01413-7.
https://www.ncbi.nlm.nih.gov/pubmed/34127854
Kwan, M. Y. W., Chua, G. T., Chow, C. B., Tsao, S. S. L., To,
K. K. W., Yuen, K. Y., . . . Ip, P. (2021). mRNA COVID vaccine and
myocarditis in adolescents. Hong Kong Med J, 27(5), 326-327.
doi:10.12809/hkmj215120. https://www.ncbi.nlm.nih.gov/pubmed/34393110
Lee, E., Chew, N. W. S., Ng, P., & Yeo, T. J. (2021).
Reply to „Letter to the editor: Myocarditis should be considered in
those with a troponin rise and unobstructed coronary arteries
following PfizerBioNTech COVID-19 vaccination”. QJM.
doi:10.1093/qjmed/hcab232.
https://www.ncbi.nlm.nih.gov/pubmed/34463770
Lee, E. J., Cines, D. B., Gernsheimer, T., Kessler, C.,
Michel, M., Tarantino, M. D., . . . Bussel, J. B. (2021).
Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination.
Am J Hematol, 96(5), 534-537. doi:10.1002/ajh.26132.
https://www.ncbi.nlm.nih.gov/pubmed/33606296
Levin, D., Shimon, G., Fadlon-Derai, M., Gershovitz, L.,
Shovali, A., Sebbag, A., . . . Gordon, B. (2021). Myocarditis
following COVID-19 vaccination – A case series. Vaccine, 39(42),
6195-6200. doi:10.1016/j.vaccine.2021.09.004.
https://www.ncbi.nlm.nih.gov/pubmed/34535317
Li, J., Hui, A., Zhang, X., Yang, Y., Tang, R., Ye, H., . . .
Zhu, F. (2021). Safety and immunogenicity of the SARS-CoV-2 BNT162b1
mRNA vaccine in younger and older Chinese adults: a randomized,
placebo-controlled, double-blind phase 1 study. Nat Med, 27(6),
1062-1070. doi:10.1038/s41591-021-01330-9.
https://www.ncbi.nlm.nih.gov/pubmed/33888900
Li, M., Yuan, J., Lv, G., Brown, J., Jiang, X., & Lu, Z.
K. (2021). Myocarditis and Pericarditis following COVID-19
Vaccination: Inequalities in Age and Vaccine Types. J Pers Med,
11(11). doi:10.3390/jpm11111106.
https://www.ncbi.nlm.nih.gov/pubmed/34834458
Lim, Y., Kim, M. C., Kim, K. H., Jeong, I. S., Cho, Y. S.,
Choi, Y. D., & Lee, J. E. (2021). Case Report: Acute Fulminant
Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus
Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary
Resuscitation. Front Cardiovasc Med, 8, 758996.
doi:10.3389/fcvm.2021.758996.
https://www.ncbi.nlm.nih.gov/pubmed/34778411
Long, S. S. (2021). Important Insights into Myopericarditis
after the Pfizer mRNA COVID-19 Vaccination in Adolescents. J Pediatr,
238, 5. doi:10.1016/j.jpeds.2021.07.057.
https://www.ncbi.nlm.nih.gov/pubmed/34332972
Luk, A., Clarke, B., Dahdah, N., Ducharme, A., Krahn, A.,
McCrindle, B., . . . McDonald, M. (2021). Myocarditis and
Pericarditis After COVID-19 mRNA Vaccination: Practical
Considerations for Care Providers. Can J Cardiol, 37(10), 1629-1634.
doi:10.1016/j.cjca.2021.08.001.
https://www.ncbi.nlm.nih.gov/pubmed/34375696
Madelon, N., Lauper, K., Breville, G., Sabater Royo, I.,
Goldstein, R., Andrey, D. O., . . . Eberhardt, C. S. (2021). Robust T
cell responses in anti-CD20 treated patients following COVID-19
vaccination: a prospective cohort study. Clin Infect Dis.
doi:10.1093/cid/ciab954. https://www.ncbi.nlm.nih.gov/pubmed/34791081
Mangat, C., & Milosavljevic, N. (2021). BNT162b2
Vaccination during Pregnancy Protects Both the Mother and Infant:
Anti-SARS-CoV-2 S Antibodies Persistently Positive in an Infant at 6
Months of Age. Case Rep Pediatr, 2021, 6901131.
doi:10.1155/2021/6901131.
https://www.ncbi.nlm.nih.gov/pubmed/34676123
Mark, C., Gupta, S., Punnett, A., Upton, J., Orkin, J.,
Atkinson, A., . . . Alexander, S. (2021). Safety of administration of
BNT162b2 mRNA (Pfizer-BioNTech) COVID-19 vaccine in youths and young
adults with a history of acute lymphoblastic leukemia and allergy to
PEG-asparaginase. Pediatr Blood Cancer, 68(11), e29295.
doi:10.1002/pbc.29295. https://www.ncbi.nlm.nih.gov/pubmed/34398511
Martins-Filho, P. R., Quintans-Junior, L. J., de Souza Araujo,
A. A., Sposato, K. B., Souza Tavares, C. S., Gurgel, R. Q., . . .
Santos, V. S. (2021). Socio-economic inequalities and COVID-19
incidence and mortality in Brazilian children: a nationwide
register-based study. Public Health, 190, 4-6.
doi:10.1016/j.puhe.2020.11.005.
https://www.ncbi.nlm.nih.gov/pubmed/33316478
McLean, K., & Johnson, T. J. (2021). Myopericarditis in a
previously healthy adolescent male following COVID-19 vaccination: A
case report. Acad Emerg Med, 28(8), 918-921. doi:10.1111/acem.14322.
https://www.ncbi.nlm.nih.gov/pubmed/34133825
Mevorach, D., Anis, E., Cedar, N., Bromberg, M., Haas, E. J.,
Nadir, E., . . . Alroy-Preis, S. (2021). Myocarditis after BNT162b2
mRNA Vaccine against Covid-19 in Israel. N Engl J Med, 385(23),
2140-2149. doi:10.1056/NEJMoa2109730.
https://www.ncbi.nlm.nih.gov/pubmed/34614328
Minocha, P. K., Better, D., Singh, R. K., & Hoque, T.
(2021). Recurrence of Acute Myocarditis Temporally Associated with
Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a
Male Adolescent. J Pediatr, 238, 321-323.
doi:10.1016/j.jpeds.2021.06.035.
https://www.ncbi.nlm.nih.gov/pubmed/34166671
Mizrahi, B., Lotan, R., Kalkstein, N., Peretz, A., Perez, G.,
Ben-Tov, A., . . . Patalon, T. (2021). Correlation of
SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun,
12(1), 6379. doi:10.1038/s41467-021-26672-3.
https://www.ncbi.nlm.nih.gov/pubmed/34737312
Moffitt, K., Cheung, E., Yeung, T., Stamoulis, C., &
Malley, R. (2021). Analysis of Staphylococcus aureus Transcriptome in
Pediatric Soft Tissue Abscesses and Comparison to Murine Infections.
Infect Immun, 89(4). doi:10.1128/IAI.00715-20.
https://www.ncbi.nlm.nih.gov/pubmed/33526560
Mohamed, L., Madsen, A. M. R., Schaltz-Buchholzer, F.,
Ostenfeld, A., Netea, M. G., Benn, C. S., & Kofoed, P. E. (2021).
Reactivation of BCG vaccination scars after vaccination with
mRNA-Covid-vaccines: two case reports. BMC Infect Dis, 21(1), 1264.
doi:10.1186/s12879-021-06949-0.
https://www.ncbi.nlm.nih.gov/pubmed/34930152
Montgomery, J., Ryan, M., Engler, R., Hoffman, D.,
McClenathan, B., Collins, L., . . . Cooper, L. T., Jr. (2021).
Myocarditis Following Immunization With mRNA COVID-19 Vaccines in
Members of the US Military. JAMA Cardiol, 6(10), 1202-1206.
doi:10.1001/jamacardio.2021.2833.
https://www.ncbi.nlm.nih.gov/pubmed/34185045
Murakami, Y., Shinohara, M., Oka, Y., Wada, R., Noike, R.,
Ohara, H., . . . Ikeda, T. (2021). Myocarditis Following a COVID-19
Messenger RNA Vaccination: A Japanese Case Series. Intern Med.
doi:10.2169/internalmedicine.8731-21.
https://www.ncbi.nlm.nih.gov/pubmed/34840235
Nagasaka, T., Koitabashi, N., Ishibashi, Y., Aihara, K.,
Takama, N., Ohyama, Y., . . . Kaneko, Y. (2021). Acute Myocarditis
Associated with COVID-19 Vaccination: A Case Report. J Cardiol Cases.
doi:10.1016/j.jccase.2021.11.006.
https://www.ncbi.nlm.nih.gov/pubmed/34876937
Ntouros, P. A., Vlachogiannis, N. I., Pappa, M., Nezos, A.,
Mavragani, C. P., Tektonidou, M. G., . . . Sfikakis, P. P. (2021).
Effective DNA damage response after acute but not chronic immune
challenge: SARS-CoV-2 vaccine versus Systemic Lupus Erythematosus.
Clin Immunol, 229, 108765. doi:10.1016/j.clim.2021.108765.
https://www.ncbi.nlm.nih.gov/pubmed/34089859
Nygaard, U., Holm, M., Bohnstedt, C., Chai, Q., Schmidt, L.
S., Hartling, U. B., . . . Stensballe, L. G. (2022). Population-based
Incidence of Myopericarditis After COVID-19 Vaccination in Danish
Adolescents. Pediatr Infect Dis J, 41(1), e25-e28.
doi:10.1097/INF.0000000000003389.
https://www.ncbi.nlm.nih.gov/pubmed/34889875
Oberhardt, V., Luxenburger, H., Kemming, J., Schulien, I.,
Ciminski, K., Giese, S., . . . Hofmann, M. (2021). Rapid and stable
mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature,
597(7875), 268-273. doi:10.1038/s41586-021-03841-4.
https://www.ncbi.nlm.nih.gov/pubmed/34320609
Park, H., Yun, K. W., Kim, K. R., Song, S. H., Ahn, B., Kim,
D. R., . . . Kim, Y. J. (2021). Epidemiology and Clinical Features of
Myocarditis/Pericarditis before the Introduction of mRNA COVID-19
Vaccine in Korean Children: a Multicenter Study. J Korean Med Sci,
36(32), e232. doi:10.3346/jkms.2021.36.e232.
https://www.ncbi.nlm.nih.gov/pubmed/34402230
Park, J., Brekke, D. R., & Bratincsak, A. (2021).
Self-limited myocarditis presenting with chest pain and ST segment
elevation in adolescents after vaccination with the BNT162b2 mRNA
vaccine. Cardiol Young, 1-4. doi:10.1017/S1047951121002547.
https://www.ncbi.nlm.nih.gov/pubmed/34180390
Patel, Y. R., Louis, D. W., Atalay, M., Agarwal, S., &
Shah, N. R. (2021). Cardiovascular magnetic resonance findings in
young adult patients with acute myocarditis following mRNA COVID-19
vaccination: a case series. J Cardiovasc Magn Reson, 23(1), 101.
doi:10.1186/s12968-021-00795-4.
https://www.ncbi.nlm.nih.gov/pubmed/34496880
Patone, M., Mei, X. W., Handunnetthi, L., Dixon, S., Zaccardi,
F., Shankar-Hari, M., . . . Hippisley-Cox, J. (2021). Risks of
myocarditis, pericarditis, and cardiac arrhythmias associated with
COVID-19 vaccination or SARS-CoV-2 infection. Nat Med.
doi:10.1038/s41591-021-01630-0.
https://www.ncbi.nlm.nih.gov/pubmed/34907393
Patrignani, A., Schicchi, N., Calcagnoli, F., Falchetti, E.,
Ciampani, N., Argalia, G., & Mariani, A. (2021). Acute
myocarditis following Comirnaty vaccination in a healthy man with
previous SARS-CoV-2 infection. Radiol Case Rep, 16(11), 3321-3325.
doi:10.1016/j.radcr.2021.07.082.
https://www.ncbi.nlm.nih.gov/pubmed/34367386
Perez, Y., Levy, E. R., Joshi, A. Y., Virk, A.,
Rodriguez-Porcel, M., Johnson, M., . . . Swift, M. D. (2021).
Myocarditis Following COVID-19 mRNA Vaccine: A Case Series and
Incidence Rate Determination. Clin Infect Dis.
doi:10.1093/cid/ciab926. https://www.ncbi.nlm.nih.gov/pubmed/34734240
Perrotta, A., Biondi-Zoccai, G., Saade, W., Miraldi, F.,
Morelli, A., Marullo, A. G., . . . Peruzzi, M. (2021). A snapshot
global survey on side effects of COVID-19 vaccines among healthcare
professionals and armed forces with a focus on headache. Panminerva
Med, 63(3), 324-331. doi:10.23736/S0031-0808.21.04435-9.
https://www.ncbi.nlm.nih.gov/pubmed/34738774
Pinana, J. L., Lopez-Corral, L., Martino, R., Montoro, J.,
Vazquez, L., Perez, A., . . . Cell Therapy, G. (2022).
SARS-CoV-2-reactive antibody detection after SARS-CoV-2 vaccination
in hematopoietic stem cell transplant recipients: Prospective survey
from the Spanish Hematopoietic Stem Cell Transplantation and Cell
Therapy Group. Am J Hematol, 97(1), 30-42. doi:10.1002/ajh.26385.
https://www.ncbi.nlm.nih.gov/pubmed/34695229
Revon-Riviere, G., Ninove, L., Min, V., Rome, A., Coze, C.,
Verschuur, A., . . . Andre, N. (2021). The BNT162b2 mRNA COVID-19
vaccine in adolescents and young adults with cancer: A monocentric
experience. Eur J Cancer, 154, 30-34. doi:10.1016/j.ejca.2021.06.002.
https://www.ncbi.nlm.nih.gov/pubmed/34233234
Sanchez Tijmes, F., Thavendiranathan, P., Udell, J. A.,
Seidman, M. A., & Hanneman, K. (2021). Cardiac MRI Assessment of
Nonischemic Myocardial Inflammation: State of the Art Review and
Update on Myocarditis Associated with COVID-19 Vaccination. Radiol
Cardiothorac Imaging, 3(6), e210252. doi:10.1148/ryct.210252.
https://www.ncbi.nlm.nih.gov/pubmed/34934954
Schauer, J., Buddhe, S., Colyer, J., Sagiv, E., Law, Y.,
Mallenahalli Chikkabyrappa, S., & Portman, M. A. (2021).
Myopericarditis After the Pfizer Messenger Ribonucleic Acid
Coronavirus Disease Vaccine in Adolescents. J Pediatr, 238, 317-320.
doi:10.1016/j.jpeds.2021.06.083.
https://www.ncbi.nlm.nih.gov/pubmed/34228985
Schneider, J., Sottmann, L., Greinacher, A., Hagen, M.,
Kasper, H. U., Kuhnen, C., . . . Schmeling, A. (2021). Postmortem
investigation of fatalities following vaccination with COVID-19
vaccines. Int J Legal Med, 135(6), 2335-2345.
doi:10.1007/s00414-021-02706-9.
https://www.ncbi.nlm.nih.gov/pubmed/34591186
Schramm, R., Costard-Jackle, A., Rivinius, R., Fischer, B.,
Muller, B., Boeken, U., . . . Gummert, J. (2021). Poor humoral and
T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2
in cardiothoracic transplant recipients. Clin Res Cardiol, 110(8),
1142-1149. doi:10.1007/s00392-021-01880-5.
https://www.ncbi.nlm.nih.gov/pubmed/34241676
Sessa, F., Salerno, M., Esposito, M., Di Nunno, N., Zamboni,
P., & Pomara, C. (2021). Autopsy Findings and Causality
Relationship between Death and COVID-19 Vaccination: A Systematic
Review. J Clin Med, 10(24). doi:10.3390/jcm10245876.
https://www.ncbi.nlm.nih.gov/pubmed/34945172
Sharif, N., Alzahrani, K. J., Ahmed, S. N., & Dey, S. K.
(2021). Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A
Systematic Review and Meta-Analysis. Front Immunol, 12, 714170.
doi:10.3389/fimmu.2021.714170.
https://www.ncbi.nlm.nih.gov/pubmed/34707602
Shay, D. K., Gee, J., Su, J. R., Myers, T. R., Marquez, P.,
Liu, R., . . . Shimabukuro, T. T. (2021). Safety Monitoring of the
Janssen (Johnson & Johnson) COVID-19 Vaccine – United States,
March-April 2021. MMWR Morb Mortal Wkly Rep, 70(18), 680-684.
doi:10.15585/mmwr.mm7018e2.
https://www.ncbi.nlm.nih.gov/pubmed/33956784
Shazley, O., & Alshazley, M. (2021). A COVID-Positive
52-Year-Old Man Presented With Venous Thromboembolism and
Disseminated Intravascular Coagulation Following Johnson &
Johnson Vaccination: A Case-Study. Cureus, 13(7), e16383.
doi:10.7759/cureus.16383.
https://www.ncbi.nlm.nih.gov/pubmed/34408937
Shiyovich, A., Witberg, G., Aviv, Y., Eisen, A., Orvin, K.,
Wiessman, M., . . . Hamdan, A. (2021). Myocarditis following COVID-19
vaccination: magnetic resonance imaging study. Eur Heart J Cardiovasc
Imaging. doi:10.1093/ehjci/jeab230.
https://www.ncbi.nlm.nih.gov/pubmed/34739045
Simone, A., Herald, J., Chen, A., Gulati, N., Shen, A. Y.,
Lewin, B., & Lee, M. S. (2021). Acute Myocarditis Following
COVID-19 mRNA Vaccination in Adults Aged 18 Years or Older. JAMA
Intern Med, 181(12), 1668-1670. doi:10.1001/jamainternmed.2021.5511.
https://www.ncbi.nlm.nih.gov/pubmed/34605853
Singer, M. E., Taub, I. B., & Kaelber, D. C. (2021). Risk
of Myocarditis from COVID-19 Infection in People Under Age 20: A
Population-Based Analysis. medRxiv. doi:10.1101/2021.07.23.21260998.
https://www.ncbi.nlm.nih.gov/pubmed/34341797
Smith, C., Odd, D., Harwood, R., Ward, J., Linney, M., Clark,
M., . . . Fraser, L. K. (2021). Deaths in children and young people
in England after SARS-CoV-2 infection during the first pandemic year.
Nat Med. doi:10.1038/s41591-021-01578-1.
https://www.ncbi.nlm.nih.gov/pubmed/34764489
Snapiri, O., Rosenberg Danziger, C., Shirman, N., Weissbach,
A., Lowenthal, A., Ayalon, I., . . . Bilavsky, E. (2021). Transient
Cardiac Injury in Adolescents Receiving the BNT162b2 mRNA COVID-19
Vaccine. Pediatr Infect Dis J, 40(10), e360-e363.
doi:10.1097/INF.0000000000003235.
https://www.ncbi.nlm.nih.gov/pubmed/34077949
Spinner, J. A., Julien, C. L., Olayinka, L., Dreyer, W. J.,
Bocchini, C. E., Munoz, F. M., & Devaraj, S. (2021). SARS-CoV-2
anti-spike antibodies after vaccination in pediatric heart
transplantation: A first report. J Heart Lung Transplant.
doi:10.1016/j.healun.2021.11.001.
https://www.ncbi.nlm.nih.gov/pubmed/34911654
Starekova, J., Bluemke, D. A., Bradham, W. S., Grist, T. M.,
Schiebler, M. L., & Reeder, S. B. (2021). Myocarditis Associated
with mRNA COVID-19 Vaccination. Radiology, 301(2), E409-E411.
doi:10.1148/radiol.2021211430.
https://www.ncbi.nlm.nih.gov/pubmed/34282971
Sulemankhil, I., Abdelrahman, M., & Negi, S. I. (2021).
Temporal association between the COVID-19 Ad26.COV2.S vaccine and
acute myocarditis: A case report and literature review. Cardiovasc
Revasc Med. doi:10.1016/j.carrev.2021.08.012.
https://www.ncbi.nlm.nih.gov/pubmed/34420869
Tailor, P. D., Feighery, A. M., El-Sabawi, B., & Prasad,
A. (2021). Case report: acute myocarditis following the second dose
of mRNA-1273 SARS-CoV-2 vaccine. Eur Heart J Case Rep, 5(8), ytab319.
doi:10.1093/ehjcr/ytab319.
https://www.ncbi.nlm.nih.gov/pubmed/34514306
Takeda, M., Ishio, N., Shoji, T., Mori, N., Matsumoto, M., &
Shikama, N. (2021). Eosinophilic Myocarditis Following Coronavirus
Disease 2019 (COVID-19) Vaccination. Circ J.
doi:10.1253/circj.CJ-21-0935.
https://www.ncbi.nlm.nih.gov/pubmed/34955479
Team, C. C.-R., Food, & Drug, A. (2021). Allergic
Reactions Including Anaphylaxis After Receipt of the First Dose of
Pfizer-BioNTech COVID-19 Vaccine – United States, December 14-23,
2020. MMWR Morb Mortal Wkly Rep, 70(2), 46-51.
doi:10.15585/mmwr.mm7002e1.
https://www.ncbi.nlm.nih.gov/pubmed/33444297
Thompson, M. G., Burgess, J. L., Naleway, A. L., Tyner, H.,
Yoon, S. K., Meece, J., . . . Gaglani, M. (2021). Prevention and
Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N
Engl J Med, 385(4), 320-329. doi:10.1056/NEJMoa2107058.
https://www.ncbi.nlm.nih.gov/pubmed/34192428
Tinoco, M., Leite, S., Faria, B., Cardoso, S., Von Hafe, P.,
Dias, G., . . . Lourenco, A. (2021). Perimyocarditis Following
COVID-19 Vaccination. Clin Med Insights Cardiol, 15,
11795468211056634. doi:10.1177/11795468211056634.
https://www.ncbi.nlm.nih.gov/pubmed/34866957
Truong, D. T., Dionne, A., Muniz, J. C., McHugh, K. E.,
Portman, M. A., Lambert, L. M., . . . Newburger, J. W. (2021).
Clinically Suspected Myocarditis Temporally Related to COVID-19
Vaccination in Adolescents and Young Adults. Circulation.
doi:10.1161/CIRCULATIONAHA.121.056583.
https://www.ncbi.nlm.nih.gov/pubmed/34865500
Tutor, A., Unis, G., Ruiz, B., Bolaji, O. A., &
Bob-Manuel, T. (2021). Spectrum of Suspected Cardiomyopathy Due to
COVID-19: A Case Series. Curr Probl Cardiol, 46(10), 100926.
doi:10.1016/j.cpcardiol.2021.100926.
https://www.ncbi.nlm.nih.gov/pubmed/34311983
Umei, T. C., Kishino, Y., Shiraishi, Y., Inohara, T., Yuasa,
S., & Fukuda, K. (2021). Recurrence of myopericarditis following
mRNA COVID-19 vaccination in a male adolescent. CJC Open.
doi:10.1016/j.cjco.2021.12.002.
https://www.ncbi.nlm.nih.gov/pubmed/34904134
Vidula, M. K., Ambrose, M., Glassberg, H., Chokshi, N., Chen,
T., Ferrari, V. A., & Han, Y. (2021). Myocarditis and Other
Cardiovascular Complications of the mRNA-Based COVID-19 Vaccines.
Cureus, 13(6), e15576. doi:10.7759/cureus.15576.
https://www.ncbi.nlm.nih.gov/pubmed/34277198
Visclosky, T., Theyyunni, N., Klekowski, N., & Bradin, S.
(2021). Myocarditis Following mRNA COVID-19 Vaccine. Pediatr Emerg
Care, 37(11), 583-584. doi:10.1097/PEC.0000000000002557.
https://www.ncbi.nlm.nih.gov/pubmed/34731877
Warren, C. M., Snow, T. T., Lee, A. S., Shah, M. M., Heider,
A., Blomkalns, A., . . . Nadeau, K. C. (2021). Assessment of Allergic
and Anaphylactic Reactions to mRNA COVID-19 Vaccines With
Confirmatory Testing in a US Regional Health System. JAMA Netw Open,
4(9), e2125524. doi:10.1001/jamanetworkopen.2021.25524.
https://www.ncbi.nlm.nih.gov/pubmed/34533570
Watkins, K., Griffin, G., Septaric, K., & Simon, E. L.
(2021). Myocarditis after BNT162b2 vaccination in a healthy male. Am
J Emerg Med, 50, 815 e811-815 e812. doi:10.1016/j.ajem.2021.06.051.
https://www.ncbi.nlm.nih.gov/pubmed/34229940
Weitzman, E. R., Sherman, A. C., & Levy, O. (2021).
SARS-CoV-2 mRNA Vaccine Attitudes as Expressed in U.S. FDA Public
Commentary: Need for a Public-Private Partnership in a Learning
Immunization System. Front Public Health, 9, 695807.
doi:10.3389/fpubh.2021.695807.
https://www.ncbi.nlm.nih.gov/pubmed/34336774
Welsh, K. J., Baumblatt, J., Chege, W., Goud, R., & Nair,
N. (2021). Thrombocytopenia including immune thrombocytopenia after
receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse
Event Reporting System (VAERS). Vaccine, 39(25), 3329-3332.
doi:10.1016/j.vaccine.2021.04.054.
https://www.ncbi.nlm.nih.gov/pubmed/34006408
Witberg, G., Barda, N., Hoss, S., Richter, I., Wiessman, M.,
Aviv, Y., . . . Kornowski, R. (2021). Myocarditis after Covid-19
Vaccination in a Large Health Care Organization. N Engl J Med,
385(23), 2132-2139. doi:10.1056/NEJMoa2110737.
https://www.ncbi.nlm.nih.gov/pubmed/34614329
Zimmermann, P., & Curtis, N. (2020). Why is COVID-19 less
severe in children? A review of the proposed mechanisms underlying
the age-related difference in severity of SARS-CoV-2 infections. Arch
Dis Child. doi:10.1136/archdischild-2020-320338.
https://www.ncbi.nlm.nih.gov/pubmed/33262177